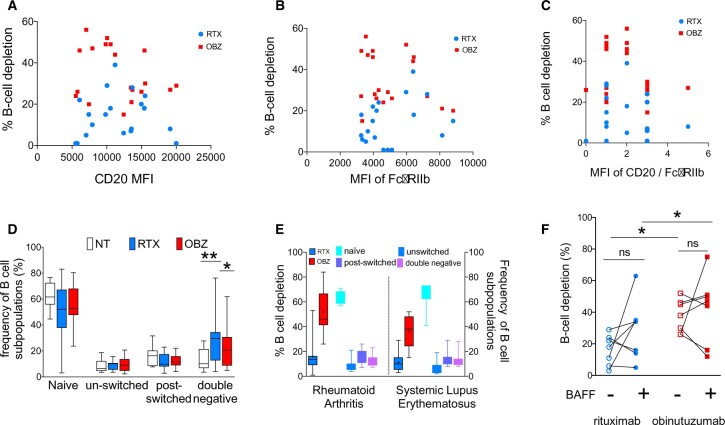
Fig. 4.
BCD in vitro with rituximab and obinutuzumab: relationship with B cell expression of CD20 and FcγRIIb; B cell subpopulations and the effect of BAFF
Relationship between percentage BCD with rituximab and obinutuzumab in whole blood assays and (A) the MFI of CD20, (B) the MFI FcγRIIb and (C) the MFI of CD20/FcγRIIb on B cells from patients with SLE (n = 19). (D) Composition of B cell subpopulations in the whole blood BCD assay. The frequency of various B cell subpopulations in samples incubated with or without mAbs in samples from patients with RA (n = 9). (E) CTI of mAbs in whole blood BCD assay and the distribution of B cell subpopulations in RA (n = 10) and SLE (n = 9) in peripheral blood prior to incubation with mAbs. (F) The effect of excess BAFF on the efficiency of BCD. Each symbol represents an individual sample and the unfilled symbols represent samples incubated without excess BAFF and the filled symbols represent samples incubated with excess BAFF. Naïve, IgD+CD27–; unswitched memory B cells, IgD+CD27+; switched memory B cells, IgD–CD27+; double negative, IgD–CD27–. BAFF: B cell activation factor; BCD: B cell depletion; CTI: cytotoxicity index; MFI, mean fluorescence intensity; NT, not treated; RTX, rituximab; OBZ, obinutuzumab; *P < 0.05 and **P < 0.001; ns: not significant.