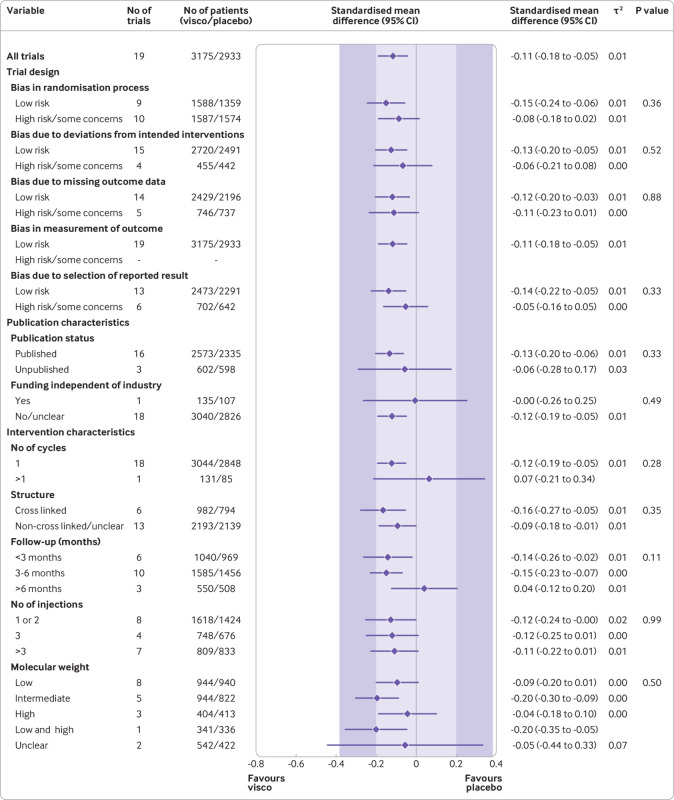
Fig 4.
Main and subgroup analyses for function. Results are based on 19 large, placebo controlled trials, including 6307 randomised participants. The shaded areas represent the areas of clinical equivalence (darker areas represent the minimal clinically important difference of 0.37, lighter areas represent the more stringent 0.2 margin of equivalence). P denotes two tailed P values for interaction (two subgroups only) or trend tests for interaction (three or more subgroups). For the molecular weight categories, the P value was based on a simple interaction test because one trial examined a preparation made of high and low molecular weight hyaluronic acids. Cycles: patients are usually given a single injection or a course of two to six injections; one cycle refers to one such course of treatment. Number of participants analysed (shown for each subgroup) might be smaller than number of randomised participants. A τ2 of up to 0.04 was prespecified to represent low heterogeneity, 0.09 to represent moderate, and 0.16 to represent high statistical heterogeneity among trial estimates.29 visco=viscosupplementation