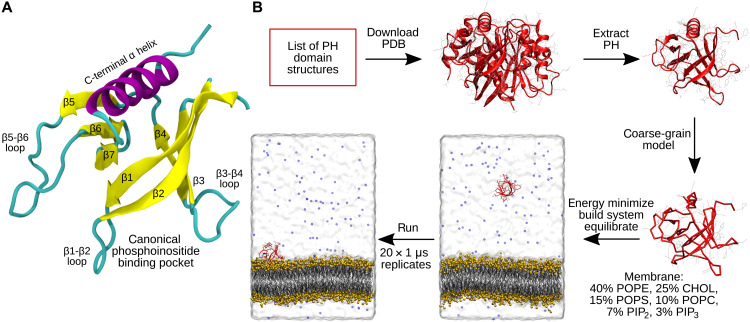
Fig. 1. Conserved PH domain structure and simulation workflow.
(A) Structure of the first PH domain of PLEK (PDB: 1xx0) demonstrates the conserved PH domain fold, consisting of a seven stranded β-barrel, capped by an α-helix, and with six variable interstrand loops. The open end of the barrel contains the canonical pocket for phosphoinositide binding. (B) Illustration of the semi-automated simulation pipeline used for high throughput PH domain simulations in this study.