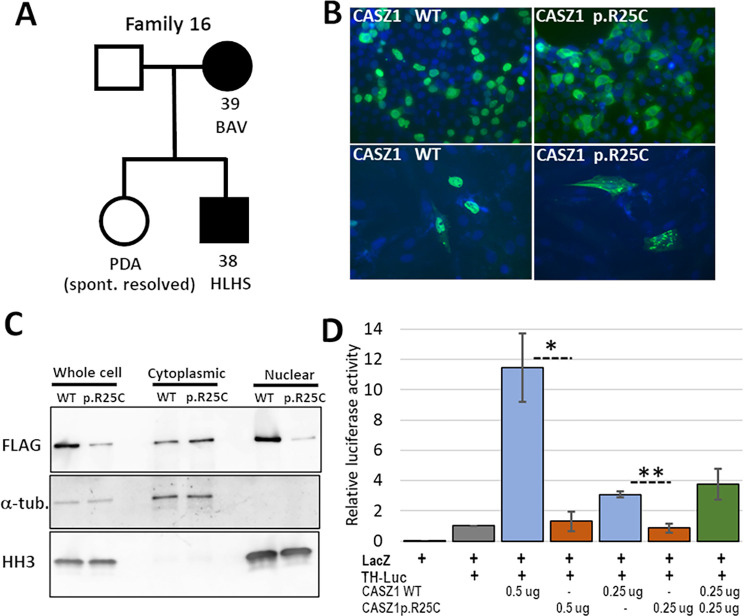
Fig 1. The CASZ1 p.R25C variant in family 16 causes mislocalization of the protein and reduced transcriptional transactivation.
A. The pedigree of family 16, showing the two affected individuals, proband [38] and his affected mother [39], for whom ES was performed. B. IF staining for FLAG in HEK293T (top) and H9c2 (bottom) cells transfected with either FLAG-tagged CASZ1 WT or FLAG-tagged CASZ1 p.R25C. Note the predominately nuclear staining in both cell types with CASZ1 WT and the increased cytoplasmic staining in cells with CASZ1 p.R25C. C. Western blot analysis of unfractionated and fractionated protein extracts from HEK293T cells transfected with either FLAG-tagged CASZ1 WT or CASZ1 p.R25C, and probed with anti-FLAG antibody to detect tagged CASZ1, a-tubulin as a cytoplasmic marker, and histone H3 (HH3) as a nuclear marker. A majority of CASZ1 WT was present in the nuclear fraction. The CASZ1 p.R25C signal was higher in the cytoplasmic fraction, consistent with the IF staining results. D. A luciferase assay, using the tyrosine hydroxyylase (TH) promoter as a target on a luciferase expression construct in HEK293T cells, showed that cotransfection of CASZ1 WT increased luciferase reporter signal by over 10-fold, while CASZ1 p.R25C did not significantly increase luciferase signal above background. The effect of CASZ1 WT was dosage sensitive (compare 0.5 mg vs 0.25 mg), and cotransfection of CASZ1 WT and p.R25C was not significantly different from CASZ1 WT alone. The graphed data represent the combined results from three independent experiments in which each condition was performed in triplicate. (Error bars indicate SEM, * p = 0.013, ** p = 0.004).