Abstract
A maternal high-fat diet (HFD) can impact the offspring’s development of liver steatosis, with fetal development in utero being a crucial period. Therefore, this study investigated the mechanism and whether butyrate can rescue liver injury caused by maternal HFD in the fetus. Pregnant female Sprague Dawley rats were randomly divided into two groups, prenatal HFD (58% fat) exposure or normal control diet (4.5% fat). The HFD group was fed an HFD 7 weeks before mating and during gestation until sacrifice at gestation 21 days. After confirmation of mating, the other HFD group was supplemented with sodium butyrate (HFSB). The results showed that maternal liver histology showed lipid accumulation with steatosis and shortened ileum villi in HFD, which was ameliorated in the HFSB group (P<0.05). There was increased fetal liver and ileum TUNEL staining and IL-6 expression with increased fetal liver TNF-α and malondialdehyde expression in the HFD group (P<0.05), which decreased in the HFSB group (P<0.05). The fetal liver expression of phospho-AKT/AKT and GPX1 decreased in the HFD group but increased in the HFSB group (P<0.05). In conclusion that oxidative stress with inflammation and apoptosis plays a vital role after maternal HFD in the fetus liver that can be ameliorated with butyrate supplementation.
Introduction
The developmental origin of health and disease is an important issue [1]. The fetus in particular is exposed to a wide range of environmental conditions, including over-nutrition [2]. Fetal development in utero is a particularly vulnerable time during which the maternal environment affects long-term fetal growth and disease development [3]. Indeed, nonalcoholic fatty liver disease can originate in early life and lead to offspring metabolic syndrome, liver cirrhosis, and end-stage liver disease [4]. Moreover, the deleterious effects of a high-fat diet (HFD) on perinatal can impair adult liver [5], so it was hypothesized that maternal HFD during pregnancy results in liver steatosis and retroperitoneal adiposity in the offspring [6, 7].
Early maternal diets can influence gut microbiota composition in the offspring through in utero maternal gut microbiota transfer [2, 8]. Furthermore, maternal HFD during pregnancy induces dysbiosis in the offspring [8], with these changes persisting until adulthood with long-term effects on metabolic fatty liver development [9]. The gut microbiota metabolite, short chain fatty acid butyrate, produced by the microbial fermentation of dietary fiber in the large intestine has multiple beneficial effects in mammals [10, 11]. Butyrate improves glucose tolerance in the rat liver by inhibiting inflammation, reducing steatosis and metabolic syndrome [12, 13]. It decreased malondialdehyde (MDA) with cellular oxidative stress to prevent liver toxicity [14], as well as increasing antioxidative enzyme activities to ameliorate liver oxidative damage and several pro-inflammatory genes [15, 16]. The rationale for the study is that oxidative stress in the liver damage induced by a maternal HFD could be recovered by butyrate.
The implications of prenatal insults on the risk of developing a disease in offspring provide a basis for the study of the development of liver steatosis in the fetus. The mechanisms by which fetal metabolic systems cope with excess nutrition during intrauterine development are important and remain relatively unexplored. Hence, this study explored whether butyrate therapy can inverse prenatal HFD stress in the fetus to provide insights for future clinical management strategies.
Materials and methods
2.1 Animals
The animal study was performed at the Animal Experimental Center of Kaohsiung Chang Gung Memorial Hospital after approval from the Institutional Animal Care and Use Committee of the Hospital (Approval No. 2019052802). Sprague Dawley (SD) rats were purchased from Lasco Laboratories (Taipei, Taiwan) and were housed in the animal facility in a 12-hr light/dark cycle with lights on at 7 a.m., and litters were checked at 10:00 am daily.
The HFD is composed of 5.56 kcal/g dry weight, 23 g/100 g protein, 35.5 g/100 g carbohydrate, and 35.8 g/100 g saturated fat (58 kcal% fat D12331) was purchased from the Research Diet Company, USA. The normal-chow diet (NCD) is composed of 3.85 kcal/g dry weight, 19.2 g/100 g protein, 67.3 g/100 g carbohydrate, and 4.3 g/100 g saturated fat. The female rats were allowed 24 h to mate with male rats, then separated from the male rats and housed individually in a standard plastic cage.
Sprague Dawley rats and their fetuses were randomly divided into groups, prenatal HFD, NCD, and HFSB (N = 6 for each group). Each group of two pregnant female rats will give birth to an average of 20 offspring. Group I NCD: SD rats were fed with an NCD before mating and during gestation till sacrifice on gestational day 21 (GD21). Group II HFD: Maternal SD rats were fed with HFD 7 weeks before mating and during gestation till sacrifice on GD21. Group III HFSB: Maternal SD rats were fed with HFD 7 weeks before mating and during gestation and the pregnant rats were administrated with 1% (w/v) sodium butyrate (B5887, SIGMA, MO, USA) in drinking water (150 mg/kg/day) via gavage [17] from GD0 until sacrifice at GD21. There were no adverse effects on gastrointestinal symptoms observed during sodium butyrate administration.
2.2 Measurement of plasma biochemistry parameters
Blood samples were collected by cardiac puncture when the rats were sacrificed to alleviate their suffering [18]. The plasma levels of total cholesterol, aspartate transaminase (AST), and alanine aminotransferase (ALT) were determined by a standard auto-analyzer (Hitachi model 7450, Tokyo, Japan).
2.3 Tissue preparation
Rats were anesthetized with 25 mg/kg Zoletil and 23 mg/kg Xylazine on GD21 until no conscious then perfused continuously with normal saline via a peristaltic pump. The maternal liver and ileum were immediately removed and kept on an ice plate. The lumen of the small intestinal tissues was cleaned using ice-cold phosphate-buffered saline solution (PBS, pH 7.4) and embedded in Swiss rolls in paraffin, followed by hematoxylin and eosin (H&E) staining for evaluation of the villi length. The liver tissue was cut into pieces and embedded in paraffin for immunohistochemistry. The remainder of the liver and ileum were dissected and stored at -80°C. The fetal liver and ileum were also collected for analysis. Though endogenous butyrate absorption by colonocytes have been well documented [19], the effect of maternal butyrate intake on the fetal ileum and liver is unknown. We therefore studied both fetal ileum and liver tissue.
2.4 H&E staining (hematoxylin and eosin)
The liver and small intestine were dissected and fixed in 4% paraformaldehyde at 4°C overnight, then dehydrated in a gradient of ethanol (70%, 75%, 85%, 90%, 95%, and 100%), hyalinized in xylene, and embedded in paraffin wax at 55°C. Sections (4 μm thick) were cut and stained with an H&E Staining Kit (ScyTek Laboratories, West Logan, USA). The histologic lesions were observed with a Leica DMI-3000 microscope equipped with a digital camera and lipid accumulation in the liver and length villus of ileum were quantified using ImageJ (Fiji version 1.8.0) [20]. The semi-quantitation of the lipid droplets was calculated using approximately 500 liver cells.
2.5 Gas chromatography-mass spectrometry (GC-MS) analysis of short chain fatty acids (SCFAs)
SCFAs in stool samples were quantified and analyzed by GC-MS (Agilent GC system 7890B, MSD system 5977B). Briefly, 15 μl of stool was acidified with 50 μl of 50% sulfuric acid and 10 μl of 2-ethylbutyric acid as internal standard, before the addition of 400 μl ether and shaken for 15 min, followed by centrifugation at 9000 rpm at 4°C for 10 min. The ether layer was removed and mixed with anhydrous sodium sulfate for dehydration before 1 μl of the sample was injected (split ratio: 5:1) into a straight glass liner and held at 240°C. Helium (1 ml/min) was used as a carrier gas in the DB-FFAP capillary column (30 m×0.25 mm×0.25 μm). The oven temperature of 80°C was initially maintained for 1 min, then increased to 150°C at a rate of 5°C/min, finally increased to 240°C at a rate of 10°C/min for 12 min. The electron ionization (70 eV and 230°C) mode was used, with all samples, standards, and blanks analyzed randomly.
2.6 Immunohistochemistry
The 4 μm-sections of formalin-fixed tissues were mounted on silanized slides, deparaffinized in xylene, and rehydrated through serial baths of alcohol to water. To eliminate endogenous peroxidase activity, the hydrated sections were treated with 3% hydrogen peroxide for 10 mins and washed in PBS. Anti-IL-6 antibody (ab6672, Abcam; Cambridge, MA, USA) was diluted 1:200 in DaVinci Green (Biocare PD900, CA). The assay was assessed by an independent researcher (YMC) and one of the authors (MMT) using the Ultravision Quanto Detection system HRP DAB kit (Thermo Scientific In., TL-060-QHD; Waltham, MA, USA). The samples were observed with a Leica DMI-3000 microscope equipped with a digital camera and staining quantified by the ImageJ (Fiji version 1.8.0) [20].
2.7 Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL)
Fixed tissues embedded in paraffin were cut into 4 μm sections and mounted on slides. Apoptosis was assessed using an apoptosis detection kit (Roche, 11684817910, Mannheim, Germany) according to the manufacturer’s instructions [7]. Cells were counted from randomly selected high-power fields (200x, 400x) from each section under light microscopy. The rates of TUNEL-positive cells were calculated using 10 random fields from each rat counted for positively stained cells in the liver and each villus of the ileum.
2.8 Western blotting
Liver and ileum specimens were homogenized in lysis buffer (iNtRON, 17081; Biotechnology, Seongnam, Korea) and centrifuged at 14,000×g at 4°C for 5 min. The total protein concentration was evaluated using Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein samples (65 μg) were separated by 6~15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked in TBST buffer with 10% non-fat milk for 1 h at room temperature, before incubation at 4°C overnight with specific primary antibodies including 1:2000 tumor necrosis factor-alpha (TNF-α, #3707; Cell Signaling, Denver, MA, USA), 1:1000 cleaved-caspase 3 (#9661, Cell Signaling, Denver, MA, USA), 1:1000 phospho-AKT (Ser 473) (#9271, Cell Signaling, Denver, MA, USA), 1:1000 AKT (#9272, Cell Signaling, Denver, MA, USA), 1:1000 Zonula occludens-1 (ZO-1) (67–7300; Thermo Fisher Scientific Inc., Waltham, MA, USA), 1:1000 Claudin-3 (ab15102; Abcam, Cambridge, MA, USA), 1:1000 Occludin (#91131, Cell Signaling, Denver, MA, USA), 1:1000 glutathione peroxidase 1 (GPX1) (ab22604, Abcam, Cambridge, MA, USA) and 1:2000 Malondialdehyde (MDA) (ab27642, Abcam, Cambridge, MA, USA), 1:5000 GAPDH (ab181602, Abcam, Cambridge, MA, USA), 1:10000 beta actin (MAB1501, Merck Millipore, Temecula, CA, USA). The membranes were incubated with secondary horseradish peroxidase-conjugated anti-rabbit antibody (1:5000, Jackson ImmunoResearch, West Grove, PA USA) or anti-mouse antibody (1:10000, Jackson ImmunoResearch, West Grove, PA USA) for 1 h at room temperature, before visualization using an ECL kit (Perkin Elmer In., NEL 105001EA; Boston, MA, USA).
2.9 Statistical analysis
Data were expressed as mean ± standard error of the mean. Continuous data of biochemical parameters (serum AST, ALT, total cholesterol, maternal and fetal body weight, liver weight), western blot and histology results were analyzed by one-way ANOVA with Tukey post-hoc tests, with a P-value <0.05 considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 12.
Results
Maternal weight, biochemical data, and fetal weight
The pregnant rat body weight, maternal net weight, and fetal body weight were all increased in the HFD group (P<0.001, P<0.001, P = 0.021, respectively) and decreased in HFSB (P = 0.002, P = 0.002, P = 0.019, respectively). The maternal net weight was calculated by subtracting the fetal weight and placental weight from the maternal weight. Maternal liver weight gain occurred in the HFD group but was not reversed in the HFSB group. The AST level increased in the HFD (P = 0.027) and decreased in the HFSB group (P = 0.008), whereas ALT was not significantly different among the three groups. The total cholesterol level was unchanged in HFD rats but decreased in the HFSB group (P = 0.042) (Table 1).
Table 1. Maternal weights, biochemical data and fetal weight of the studied groups.
| BW (g) | Liver (g) | Liver / BW | Maternal net weight (g) | Fetal BW (g) | AST (U/L) | ALT (U/L) | T-Cholesterol (mg/dL) | |
|---|---|---|---|---|---|---|---|---|
| NCD | 327.6 ± 6.6#† | 8.60 ± 0.3#† | 0.026 ± 0.01#† | 288.5 ± 6.3#† | 2.83 ± 0.25#† | 85.9 ± 10.3#† | 34.6 ± 1.6 | 62.6 ± 7.3† |
| HFD | 405.1 ± 11.4*† | 13.77 ± 0.6* | 0.03 ± 0.02* | 355.0 ± 12.2*† | 3.35 ± 0.42*† | 100.6 ± 9.2*† | 34.4 ± 2.0 | 60.7 ± 8.1† |
| HFSB | 373.1 ± 10.1*# | 12.12 ± 0.7* | 0.03 ± 0.02* | 311.1 ± 7.8*# | 2.75 ± 0.16*# | 79.3 ± 10.5*# | 34.8 ± 2.2 | 50.1 ± 6.7*# |
Data were presented as mean ± standard error.
AST: aspartate transaminase, ALT: alanine aminotransferase, BW: body weight, Maternal net weight: calculated by subtraction of fetal weight and placental weight from maternal weight, T-cholesterol: total cholesterol, NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet co-treated with butyrate during gestation
* p < 0.05 compared to NCD;
# p < 0.05 compared to HFD;
† p < 0.05 compared to HFSB treated.
Maternal liver and ileum histology
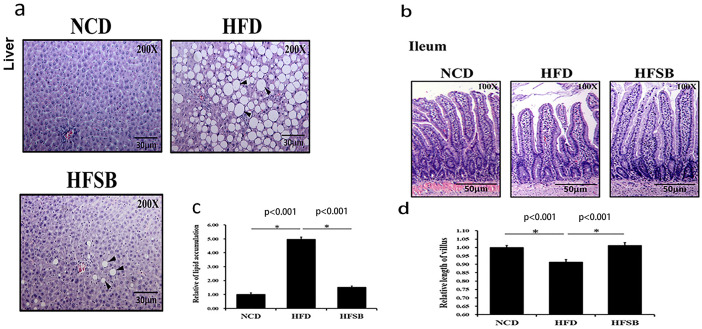
The liver histology of pregnant rats demonstrated steatosis with increased lipid accumulation (lipid droplets) in the HFD group, whereas there was decreased lipid accumulation in HFSB (Fig 1a and 1c) The shortened ileum villi with maternal HFD in pregnant rats recovered in the HFSB group (Fig 1b and 1d).
Fig 1. Histological analysis of maternal liver and ileum.
(a) H&E staining in the liver and (b) ileal tissue. Semi-quantitative analysis of (c) hepatic lipid accumulation and (d) ileal villous length. NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet with butyrate supplementation during pregnancy (n = 6), * p < 0.05. Black arrowheads: lipid droplets.
Maternal stool SCFAs
There was no significant difference among the three groups regarding maternal stool levels of acetic acid levels (Fig 2a). The maternal stool levels of propionic acid and butyric acid decreased in HFD (Fig 2b and 2c), while only butyric acid increased in HFSB (Fig 2c).
Fig 2. Short chain fatty acids profile of maternal rats.
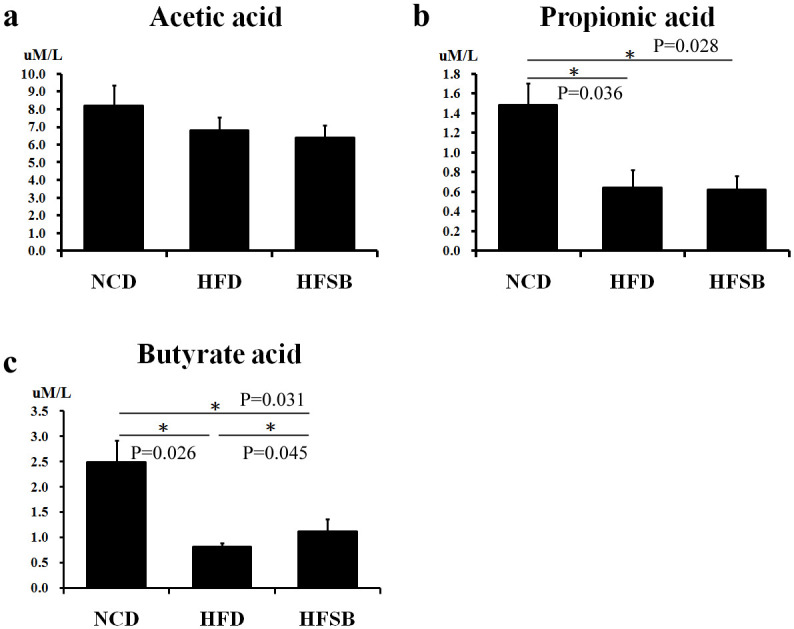
Maternal stool levels of (a) acetic acid, (b) propionic acid, (c) and butyric acid. NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet with butyrate supplementation during pregnancy (n = 6), * p < 0.05.
Fetal liver and ileum inflammation
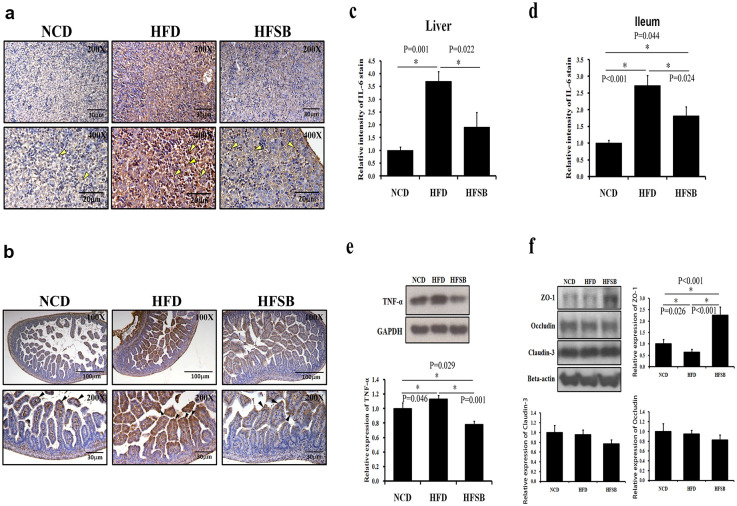
IL-6 and TNF-α was used as inflammation markers [21]. IL-6 staining increased in the fetal liver and ileum of HFD but decreased in HFSB (Fig 3a–3d). Similarly, western blotting revealed increased TNF-α expression in the fetal liver of the HFD group and decreased TNF-α expression in HFSB (Fig 3e). ZO-1, occludin and claudin-3 are essential for mucosal repair, the ZO-1, occludin or claudin-3 down-regulation may be indicate a cause of poor mucosal healing [22, 23]. The fetal ileum tight junction ZO-1 expression decreased in HFD and increased in HFSB (Fig 3f). While, there was no significant difference of occludin and claudin-3 expressions among the three groups (Fig 3f).
Fig 3. Fetal liver and ileum inflammation.
(a) IL-6 staining in fetal liver and (b) fetal ileum. (c, d) Semi-quantitative analysis of IL-6 staining in fetal liver and fetal ileum, (e) western blotting of TNF-α expression in fetal liver, and (f) western blotting of fetal ileum tight junction ZO-1, occludin and claudin-3 expressions. NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet with butyrate supplementation during pregnancy (n = 6), * p < 0.05. Arrowheads: positive IL-6 staining cells.
Fetal liver and ileum apoptosis
TUNEL staining, cleaved-caspase 3.
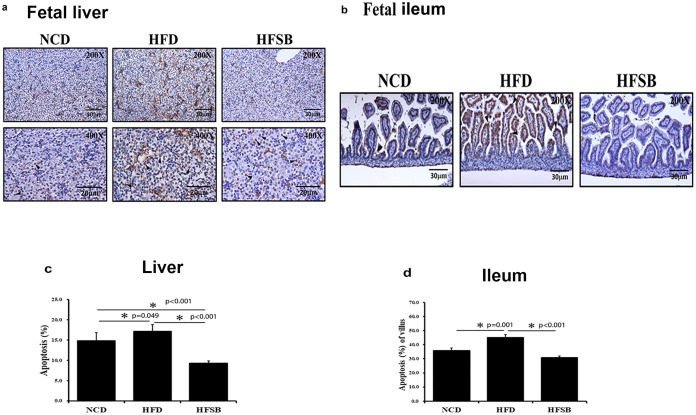
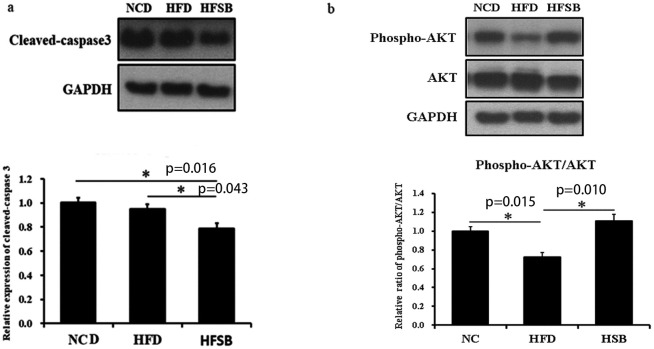
TUNEL staining indicates the activation of apoptotic pathways and was increased in the fetal liver and ileum in the HFD group but decreased in the HFSB group (Fig 4a–4d). AKT, and caspase-3 have been identified to play roles in liver cell apoptosis [24, 25]. Western blotting revealed decreased cleaved-caspase 3 expression in the fetal liver of HFSB than HFD, though it did not increase in HFD compare to NCD, the high cleaved-caspase 3 expression may be due to the anesthesia and operation process when sacrificed in NCD (Fig 5a). Phosphor-AKT was studied as an anti-apoptosis marker. The phosphor-AKT/ AKT expression decreased in HFD and increased in HFSB (Fig 5b).
Fig 4. Apoptosis in the fetal liver and ileum.
(a) TUNEL staining in the fetus liver and (b) fetus ileum. (c, d) Semi-quantitative analysis of fetus liver and ileum TUNEL stained positive cells. NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet with butyrate supplementation during pregnancy (n = 6), * p < 0.05. Black arrowheads: positive TUNEL stained cells.
Fig 5. Western blotting of apoptotic protein expression in fetal liver.
(a) cleaved-caspase 3, (b) phosphor-AKT/AKT. NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet with butyrate supplementation during pregnancy (n = 6), * p < 0.05.
Fetal liver oxidative stress
Lipid peroxidation and anti-oxidant enzyme.
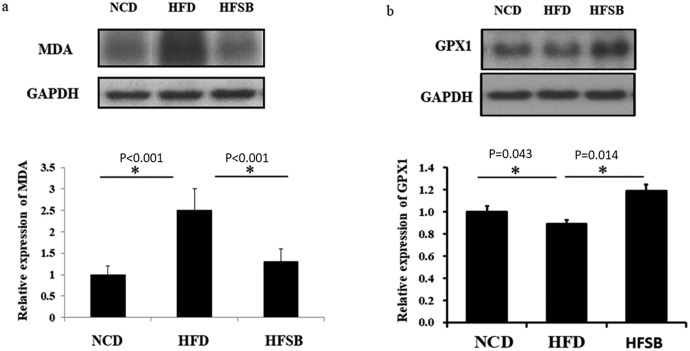
The oxidative stress of lipid peroxidation as indicated by MDA expression was increased in the fetal liver in the HFD group but decreased in HFSB (Fig 6a). Also, antioxidant enzyme, GPX1 expression in fetal liver was decreased in HFD and increased in the HFSB group (Fig 6b).
Fig 6. Fetal liver oxidative stress.
(a) western blotting of MDA expression and (b) antioxidative stress GPX1 expression. NCD: normal-chow diet, HFD: high-fat diet, HFSB: high-fat diet with butyrate supplementation during pregnancy (n = 6), * p < 0.05.
Discussion
Although there was no fetal liver steatosis or shortened villi in the fetal ileum after maternal HFD in this study, our results indicate that prenatal butyrate administration may alleviate HFD-induced liver steatosis and villi shortening in the ileum of pregnant rats, potentially reversing inflammation and apoptosis in fetal rat liver and ileum. The prenatal administration of butyrate reversed the HFD-induced body weight gain in pregnant rats, AST level, and fetal body weight gain. Prenatal butyrate administration also increased maternal stool butyric acid levels, increased the expression of fetal ileum tight junction proteins, and reduced the oxidative stress in the fetal liver.
It was reported that maternal HFD had offspring with higher weight gain [2, 21, 26] but no significant difference in fetal weight gain between obese mothers and non-obese mothers [3]. The fetus weight after maternal HFD and butyrate administration has not been studied. In this study, the maternal HFD increased maternal body weight and fetal body weight, which were reversed by prenatal butyrate supplementation. Raso et al. reported that the circulating levels of AST, ALT, and cholesterol increased at 6 weeks of HFD male SD rats and decreased after butyrate supplementation [12]. In this study, the AST increased in HFD pregnant mothers, but there was no change in ALT and cholesterol levels. These differences may be due to gender and pregnancy effects. Raso et al. also reported that butyrate supplementation reduced HFD male rat liver steatosis and inflammation [12]. The present study showed maternal liver lipid accumulation in prenatal HFD, which recovered in HFSB. The prenatal butyrate administration alleviated HFD-induced inflammation and apoptosis in the fetal rat liver.
Previous reports have shown that HFD led to reduced small intestinal villus length in a rodent model [27, 28]. Butyrate supplementation increased jejunal villus height and villus surface area [29]. The gut liver axis activation is changed after HFD. Similarly, the present study showed that pregnant mothers fed an HFD have shorter ileum villi, which recovered after butyrate administration. Nonetheless, no shortened villi were observed in the fetal ileum.
HFD can alter the microbiota composition [27] and prenatal HFD can also change the microbiota in the offspring’s stools [30]. HFD-driven dysbiosis has been linked to chronic low-grade intestinal inflammation and gut barrier dysfunction [28, 31]. There was lower species richness in the HFD group but this richness did not change after butyrate supplementation [31]. However, it was not possible to collect fetal stool samples for the microbiota study at this GD21 stage.
Maternal stool SCFAs
Dysbiosis with increased intestinal permeability may induce inflammation in the liver tissue and lead to liver steatosis [32]. Microbiota-derived metabolites such as SCFAs, act as signals to the liver [32]. Although there was no difference between stool acetic acid, our study showed decreased stool butyric acid and propionic acid compared to NCD, which is compatible with dysbiosis and liver damage under HFD. It has been reported that the intake of butyrate did not change the level of SCFAs in stools [13], whereas stool butyric acid increased after butyrate supplementation compared to the HFD group in the present study.
Propionic acid is an SCFA, a common food preservative and metabolic end-product of enteric bacteria in the gut [33]. It inhibits hepatocyte oxidation and interferes with oxidative metabolism in intact hepatocytes [34], thereby affecting liver fatty degeneration [35] and promotes hepatic damage in rats [33]. Our study showed that stool propionic acid did not increase after butyrate supplementation; however, this needs to be clarified in further studies.
Fetal liver and ileum inflammation
Many animal trials have demonstrated that maternal HFD contributes to increased fetal and offspring liver inflammation and fatty liver [36, 37]. Inflammatory cytokines may facilitate the transfer of internalized microbes from mother to offspring [26]. So, the reduced excess maternal inflammation may be a promising target for preventing adverse fetal metabolic outcomes [21]. Usually, the expression of IL-6 in hepatocytes is positively associated with the degree of inflammation [21, 38]. It was reported that HFD induced a significant increase in hepatic IL-6 mRNA, and butyrate treatment significantly prevented inflammation transcription [12]. It was also reported that up-regulation of inflammatory mediators, such as TNF-α, was found in the fatty liver [21, 39]. Butyrate supplementation suppressed several pro-inflammatory genes [15] and reduced TNF-α production [12, 13]. In our study, inflammation increased in the fetal liver as evidenced with increased IL-6, TNF-α expressions and ileum with increased IL-6 expression after maternal HFD, and the inflammation decreased with maternal butyrate supplementation. It was reported that HFD mothers developed nonalcoholic fatty liver disease in the fetal offspring [36]. A murine study showed that intestinal IL-6 expression positively correlated with intestinal permeability [40], shortened intestinal villi length after HFD with disrupted epithelium barrier function [28]. However, in this study, no liver lipid accumulation and shortened ileum villi were observed in the HFD fetus. But decreased tight junction protein expression and increased inflammation after HFD in the fetal ileum, which was reversed by butyrate supplementation. These results suggest that butyrate supplementation at this stage is more important to prevent offspring liver and ileum inflammation, as well as ileum tight junction insult.
Fetus liver and ileum apoptosis
Xie reported an increased proportion of apoptotic cells in the intestines of HFD female mice [28] and maternal HFD increased fetal hepatic apoptosis in female Japanese macaques [41]. We found activation of apoptotic pathways in the rat fetal liver and ileum with more TUNEL staining in the HFD group which could be recovered in the HFSB group. Li reported that butyrate treatment decreased caspase-3 and caspase-9 protein levels in mammary epithelial cells [16]. Similarly, the present study showed no increase in cleaved-caspase 3 in the HFD group but it was decreased in HFSB compared to HFD. It was reported that HFD increased phosphor-AKTS473 in steatohepatitis [42], and Lee reported that decreased liver steatosis increased phosphor-AKTS473 expression [43], while Li showed that butyrate treatment increased phospho-AKTS473 protein levels in epithelial cells [16]. In our study, phosphor-AKT/AKT expression decreased in the fetal liver in HFD and reversed in HFSB.
Fetal liver oxidative stress
Pregnancy with acute fatty liver is an uncommon but clinically severe hepatopathy, and oxidative stress-mediated apoptosis may be its key pathogenesis [44]. Fetal offspring from HFD mothers showed increased hepatic oxidative stress with 4-hydroxy-2-nonenal early in the third trimester [36]. Oxidative stress is the imbalance between the production of reactive oxygen species generated in the aerobic metabolism and their elimination by antioxidant defense enzymes [39]. The stress in the fatty liver may lead to substantial damage to cell structure include lipids and liver cirrhosis, with free radicals reacting with lipids to produce hydroperoxides, which can generate reactive intermediates, such as MDA, to cause cell death. [45–47] Fatty liver shows enhanced oxidative stress which may increase MDA with lipid peroxidation and decreased GPX [48, 49]. The supplementation of butyrate after HFD increased the antioxidants and decreased liver inflammation, as well as improving intestinal inflammation, oxidative stress, and epithelial defense barrier [12, 15]. In our study, GPX1 expression in the fetal liver decreased in HFD and increased in HFSB, with increased MDA production in the fetal liver, which recovered in HFSB. Taken together, this suggests that oxidative stress is increased in the fetal liver and antioxidative enzyme activity decreased after maternal HFD, which can be reversed with prenatal butyrate supplementation.
What the current work add to the existing knowledge
Butyrate supplementation had a reduction of liver steatosis and inflammation in HFD fed animals [12], and had a trend to protect HFD-induced intestinal barrier impairments [31]. There were no studies investigated the effect of prenatal butyrate in the fetal liver or fetal intestine after maternal HFD. The current study revealed anti-inflammatory and anti-apoptotic effects of prenatal butyrate on the fetal liver and intestine, the decrease in ileal villous length in HFD dams was also reversed.
Limitations
This study has some limitations. Differences in insulin-sensitive or resistant pregnant mothers were not assessed in this research. It was difficult to collect fetal stool samples, so the status of dysbiosis could not be confirmed in the fetus. The effect of prenatal butyrate administration on offspring after delivery also requires further investigation.
In conclusion, maternal HFD can cause liver inflammation, liver steatosis, and gut microbiota dysbiosis in pregnant mothers, playing a vital role in the fetal liver via oxidative stress with inflammation. Prenatal butyrate may ameliorate the degree of inflammation, oxidative stress, and apoptosis in both the mother and fetus.
Supporting information
(PDF)
Acknowledgments
The authors thank Editage for English editing and Mr. Yao-Ming Chou for the support of the experimental process consultation. We appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- GPX1
glutathione peroxidase 1
- HFD
high-fat diet
- HFSB
high-fat diet co-treated with butyrate during pregnancy
- MDA
malondialdehyde
- NCD
normal-chow diet
- SCFAs
short chain fatty acids
- TNF-α
tumor necrosis factor-alpha
- TUNEL
TdT-mediated dUTP Biotin Nick-End Labeling
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The source of this study is supported with CMRPG8J0881 and CMRPG8J0882 from the Chang Gung Memorial Hospital, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haugen AC, Schug TT, Collman G, Heindel JJ: Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis 2015, 6(2):55–64. doi: 10.1017/S2040174414000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozyrskyj AL, Kalu R, Koleva PT, Bridgman SL: Fetal programming of overweight through the microbiome: boys are disproportionately affected. J Dev Orig Health Dis 2016, 7(1):25–34. doi: 10.1017/S2040174415001269 [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S: Fetuses of obese mothers develop insulin resistance in utero. Diabetes care 2009, 32(6):1076–1080. doi: 10.2337/dc08-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumbaugh DE, Friedman JE: Developmental origins of nonalcoholic fatty liver disease. Pediatr Res 2014, 75(1–2):140–147. doi: 10.1038/pr.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parente LB, Aguila MB, Mandarim-de-Lacerda CA: Deleterious effects of high-fat diet on perinatal and postweaning periods in adult rat offspring. Clin Nutr 2008, 27(4):623–634. doi: 10.1016/j.clnu.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Tsai TA, Tsai CK, Huang LT, Sheen JM, Tiao MM, Tain YL, et al.: Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. International journal of environmental research and public health 2020, 17(8):2780. doi: 10.3390/ijerph17082780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiao MM, Lin YJ, Yu HR, Sheen JM, Lin IC, Lai YJ, et al.: Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids in health and disease 2018, 17(1):178. doi: 10.1186/s12944-018-0824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangild PT: Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 2006, 231(11):1695–1711. doi: 10.1177/153537020623101106 [DOI] [PubMed] [Google Scholar]
- 9.Klurfeld DM: Nutritional regulation of gastrointestinal growth. Frontiers in bioscience: a journal and virtual library 1999, 4:D299–302. doi: 10.2741/klurfeld [DOI] [PubMed] [Google Scholar]
- 10.Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL, et al.: Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Experimental & molecular medicine 2018, 50(12):157. doi: 10.1038/s12276-018-0183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, et al.: Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World journal of gastroenterology 2017, 23(1):60–75. doi: 10.3748/wjg.v23.i1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, et al.: Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PloS one 2013, 8(7):e68626. doi: 10.1371/journal.pone.0068626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Ding Y, Xu H, Shen C, Chen X, Li C: Effects of sodium butyrate supplementation on inflammation, gut microbiota, and short-chain fatty acids in Helicobacter pylori-infected mice. Helicobacter 2021, 26(2):e12785. doi: 10.1111/hel.12785 [DOI] [PubMed] [Google Scholar]
- 14.Pirozzi C, Lama A, Annunziata C, Cavaliere G, De Caro C, Citraro R, et al.: Butyrate prevents valproate-induced liver injury: In vitro and in vivo evidence. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2020, 34(1):676–690. doi: 10.1096/fj.201900927RR [DOI] [PubMed] [Google Scholar]
- 15.Chriett S, Dabek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L: Prominent action of butyrate over beta-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Scientific reports 2019, 9(1):742. doi: 10.1038/s41598-018-36941-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Wang HH, Nie XT, Jiang WR, Zhang YS: Sodium butyrate ameliorates lipopolysaccharide-induced cow mammary epithelial cells from oxidative stress damage and apoptosis. Journal of cellular biochemistry 2018, 120(2):2370–2381. doi: 10.1002/jcb.27565 [DOI] [PubMed] [Google Scholar]
- 17.Sun B, Jia Y, Hong J, Sun Q, Gao S, Hu Y, et al.: Sodium Butyrate Ameliorates High-Fat-Diet-Induced Non-alcoholic Fatty Liver Disease through Peroxisome Proliferator-Activated Receptor alpha-Mediated Activation of beta Oxidation and Suppression of Inflammation. Journal of agricultural and food chemistry 2018, 66(29):7633–7642. doi: 10.1021/acs.jafc.8b01189 [DOI] [PubMed] [Google Scholar]
- 18.Huang YH, Chen CJ, Tang KS, Sheen JM, Tiao MM, Tain YL, et al.: Postnatal High-Fat Diet Increases Liver Steatosis and Apoptosis Threatened by Prenatal Dexamethasone through the Oxidative Effect. Int J Mol Sci 2016, 17(3):369. doi: 10.3390/ijms17030369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al.: Butyrate: A Double-Edged Sword for Health? Advances in nutrition 2018, 9(1):21–29. doi: 10.1093/advances/nmx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varghese F, Bukhari AB, Malhotra R, De A: IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 2014, 9(5):e96801. doi: 10.1371/journal.pone.0096801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE: Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PloS one 2013, 8(6):e67791. doi: 10.1371/journal.pone.0067791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, et al.: The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021. doi: 10.1053/j.gastro.2021.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Guo R, Zhang M, Chen P, Li J, Sun Y: Protective effect of emodin on intestinal epithelial tight junction barrier integrity in rats with sepsis induced by cecal ligation and puncture. Experimental and therapeutic medicine 2020, 19(6):3521–3530. doi: 10.3892/etm.2020.8625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang M, Li C, Liu Q, Wang A, Lei M: Inhibiting Ceramide Synthesis Attenuates Hepatic Steatosis and Fibrosis in Rats With Non-alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne) 2019, 10:665. doi: 10.3389/fendo.2019.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada N, Hatano E, Koizumi N, Nitta T, Yoshida M, Yamamoto N, et al.: Akt activation protects rat liver from ischemia/reperfusion injury. J Surg Res 2004, 121(2):159–170. doi: 10.1016/j.jss.2004.04.016 [DOI] [PubMed] [Google Scholar]
- 26.Gohir W, Ratcliffe EM, Sloboda DM: Of the bugs that shape us: maternal obesity, the gut microbiome, and long-term disease risk. Pediatr Res 2015, 77(1–2):196–204. doi: 10.1038/pr.2014.169 [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Keirsey KI, Kirkland R, Grunewald ZI, Fischer JG, de La Serre CB: Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. The Journal of nutrition 2018, 148(2):209–219. doi: 10.1093/jn/nxx027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y, Ding F, Di W, Lv Y, Xia F, Sheng Y, et al.: Impact of a highfat diet on intestinal stem cells and epithelial barrier function in middleaged female mice. Molecular medicine reports 2020, 21(3):1133–1144. doi: 10.3892/mmr.2020.10932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbasi Arabshahi H, Ghasemi HA, Hajkhodadadi I, Khaltabadi Farahani AH: Effects of multicarbohydrase and butyrate glycerides on productive performance, nutrient digestibility, gut morphology, and ileal microbiota in late-phase laying hens fed corn- or wheat-based diets. Poultry science 2021, 100(5):101066. doi: 10.1016/j.psj.2021.101066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Qi C, et al.: The programming effects of nutrition-induced catch-up growth on gut microbiota and metabolic diseases in adult mice. MicrobiologyOpen 2016, 5(2):296–306. doi: 10.1002/mbo3.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Knotts TA, Goodson ML, Barboza M, Wudeck E, England G, et al.: Metabolic Responses to Butyrate Supplementation in LF- and HF-Fed Mice Are Cohort-Dependent and Associated with Changes in Composition and Function of the Gut Microbiota. Nutrients 2020, 12(11):3524. doi: 10.3390/nu12113524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dornas W, Lagente V: Intestinally derived bacterial products stimulate development of nonalcoholic steatohepatitis. Pharmacological research 2019, 141:418–428. doi: 10.1016/j.phrs.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 33.Al-Daihan S, Shafi Bhat R: Impact of Propionic Acid on Liver Damage in Rats. International journal of molecular and cellular medicine 2015, 4(3):188–195. [PMC free article] [PubMed] [Google Scholar]
- 34.Brass EP, Fennessey PV, Miller LV: Inhibition of oxidative metabolism by propionic acid and its reversal by carnitine in isolated rat hepatocytes. The Biochemical journal 1986, 236(1):131–136. doi: 10.1042/bj2360131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasgow AM, Chase HP: Effect of propionic acid on fatty acid oxidation and ureagenesis. Pediatr Res 1976, 10(7):683–686. doi: 10.1203/00006450-197607000-00010 [DOI] [PubMed] [Google Scholar]
- 36.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al.: Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. The Journal of clinical investigation 2009, 119(2):323–335. doi: 10.1172/JCI32661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kislal S, Shook LL, Edlow AG: Perinatal exposure to maternal obesity: Lasting cardiometabolic impact on offspring. Prenatal diagnosis 2020, 40(9):1109–1125. doi: 10.1002/pd.5784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE: Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008, 103(6):1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x [DOI] [PubMed] [Google Scholar]
- 39.Li S, Hong M, Tan HY, Wang N, Feng Y: Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxidative medicine and cellular longevity 2016, 2016:4234061. doi: 10.1155/2016/4234061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki T, Yoshinaga N, Tanabe S: Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. The Journal of biological chemistry 2011, 286(36):31263–31271. doi: 10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, et al.: Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One 2011, 6(2):e17261. doi: 10.1371/journal.pone.0017261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L, Tang J, Xiong X, Dong H, Huang J, Zhou S, et al.: Psoralea corylifolia L. Attenuates Nonalcoholic Steatohepatitis in Juvenile Mouse. Frontiers in pharmacology 2017, 8:876. doi: 10.3389/fphar.2017.00876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee J, Lee HY, et al.: Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology 2015, 62(1):135–146. doi: 10.1002/hep.27804 [DOI] [PubMed] [Google Scholar]
- 44.Tang W, Huang Z, Wang Y, Bo H, Fu P: Effect of plasma exchange on hepatocyte oxidative stress, mitochondria function, and apoptosis in patients with acute fatty liver of pregnancy. Artif Organs 2012, 36(3):E39–47. doi: 10.1111/j.1525-1594.2011.01417.x [DOI] [PubMed] [Google Scholar]
- 45.Ray PD, Huang BW, Tsuji Y: Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling 2012, 24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atiba AS, Abbiyesuku FM, Oparinde DP, Niran-Atiba TA, Akindele RA: Plasma Malondialdehyde (MDA): An Indication of Liver Damage in Women with Pre-Eclamsia. Ethiopian journal of health sciences 2016, 26(5):479–486. doi: 10.4314/ejhs.v26i5.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L: Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2005, 827(1):76–82. doi: 10.1016/j.jchromb.2005.06.035 [DOI] [PubMed] [Google Scholar]
- 48.Samy W, Hassanian MA: Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab journal of gastroenterology: the official publication of the Pan-Arab Association of Gastroenterology 2011, 12(2):80–85. doi: 10.1016/j.ajg.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 49.Dawood MAO, Noreldin AE, Sewilam H: Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicology and environmental safety 2021, 220:112412. doi: 10.1016/j.ecoenv.2021.112412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.