Abstract
Background
Annual mass drug administration (MDA) using praziquantel is the cornerstone of schistosomiasis morbidity control but is not sufficient to interrupt transmission. We implemented a cluster-randomized trial to compare the effectiveness of 4 different intervention packages to interrupt transmission of Schistosoma haematobium in a seasonal transmission setting of Côte d’Ivoire.
Methods
Sixty-four localities with a S. haematobium prevalence in school children aged 13–14 years above 4% were randomly assigned to 1 of 4 intervention arms over a 3-year period: (1) the current standard strategy consisting of annual MDA before peak of transmission, (2) annual MDA after peak of transmission, (3) biannual MDA, and (4) standard MDA combined with snail control. The primary outcome was prevalence and intensity of S. haematobium infection in children aged 9–12 years 1 year after the final intervention, using urine filtration performed by experienced microscopists.
Results
By study end, we observed the lowest S. haematobium prevalence in the biannual MDA, compared to the standard treatment arm (0.6% vs 7.5%; odds ratio [OR] = 0.07, 95% confidence interval [CI] = .02 to .24). The prevalence in arms 2 and 4 was about 3.5%, which was not statistically significantly different from the standard strategy (both ORs 0.4, 95% CI = .1 to ~1.8). New cases of infection were still observed in all arms at study end.
Conclusions
Biannual MDA was the only regimen that outperformed the standard treatment. All strategies resulted in decreased prevalence of infection; however, none of them was able to interrupt transmission of S. haematobium within a 3-year period.
Clinical Trials Registration
ISRCTN10926858.
Keywords: schistosomiasis, Schistosoma haematobium, seasonal transmission, interruption of transmission, Côte d’Ivoire
Four different intervention packages substantially reduced the prevalence and intensity of Schistosoma haematobium infection in a seasonal transmission area. However, none achieved interruption of transmission. Snail control failed to boost the impact of preventive chemotherapy in this 3-year cluster-randomized trial.
Schistosomiasis is a neglected tropical disease causing a considerable public health burden [1, 2]. It primarily occurs in tropical and subtropical areas of Africa, where, in 2002, an estimated 436 and 393 million people were affected by Schistosoma haematobium and Schistosoma mansoni, respectively [3]. Preventive chemotherapy with praziquantel is the mainstay of the global control strategy [4]. With partners supporting a roadmap put forth by the World Health Organization (WHO) [5], considerable progress has been made over the past 15 years [6]. New goals were set by WHO in line with World Health Assembly resolution 65.21 [7]; namely, (i) schistosomiasis elimination as a public health problem (prevalence of heavy infections <1%) and (ii) interruption of transmission (zero new cases of infection) in selected areas by 2025 [6]. Moving toward elimination might require a combination of preventive chemotherapy with other measures, such as snail control, water, sanitation, and hygiene; information, education, and communication [8–10].
The Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) supported 2 large cluster-randomized trials in Côte d’Ivoire. A first trial aimed at sustaining the control of S. mansoni with different mass drug administration (MDA) schemes [11–13]. The primary aim of the trial reported here was to assess the effectiveness of different schedules of MDA with or without snail control in interrupting S. haematobium transmission in settings characterized by seasonal transmission. The difference in prevalence and intensity of S. haematobium infection between the study arms was evaluated after 3 years of intervention in children aged 9–12 years, as this age group is considered at high risk of schistosomiasis. Elimination as public health problem and the potential added value of snail control were explored as secondary objectives. In addition, effects of interventions were assessed on first-grade children (aged 5–8 years) and adults (aged 20–55 years).
METHODS
Ethics Statement
Approval for the study was obtained from the ethics committees in Côte d’Ivoire (Comité National d’Éthique et de la Recherche; reference no. 113/MSLS/CNER-dkn, by 22 January 2015) and Switzerland (Ethikkommission Nordwest- und Zentralschweiz; reference no. UBE-15/34, by 15 April 2015). For the use of the molluscicide niclosamide, the study obtained approval of the Direction Générale des Productions et de la Sécurité Alimentaire (reference no. 0163/MINAGRI/DGPSA/DPVCQ, by 27 January 2015).
The trial was registered at the International Standard Randomized Controlled Trial Number (ISRCTN) by 21 December 2016 (reference no. ISRCTN10926858). Written informed consent was obtained from adults and parents/guardians of children aged 5–14 years. Participation was voluntary, and data were kept confidential by using participants’ individual codes instead of the names.
Study Area and Population
Details on the study populations and the eligibility criteria have been described elsewhere [14, 15]. In brief, the eligibility criteria of the study villages were: (i) location in a S. haematobium seasonal transmission area; (ii) presence of a primary school attended by at least 100 pupils aged 9–12 years; and (iii) S. haematobium prevalence of at least 4% among 50 screened children aged 13–14 years. The northern and central parts of Côte d’Ivoire were eligible, considering the marked seasonality consisting in a rainy season occurring from April to October and a dry season from November to March, an annual average temperature above 25°C and an average annual precipitation that ranges from 1115 to 1260 mm. The main activity of people in this part of Côte d’Ivoire is subsistence farming. Prior studies carried out in northern and central Côte d’Ivoire revealed low or moderate endemicity of S. haematobium [16–18].
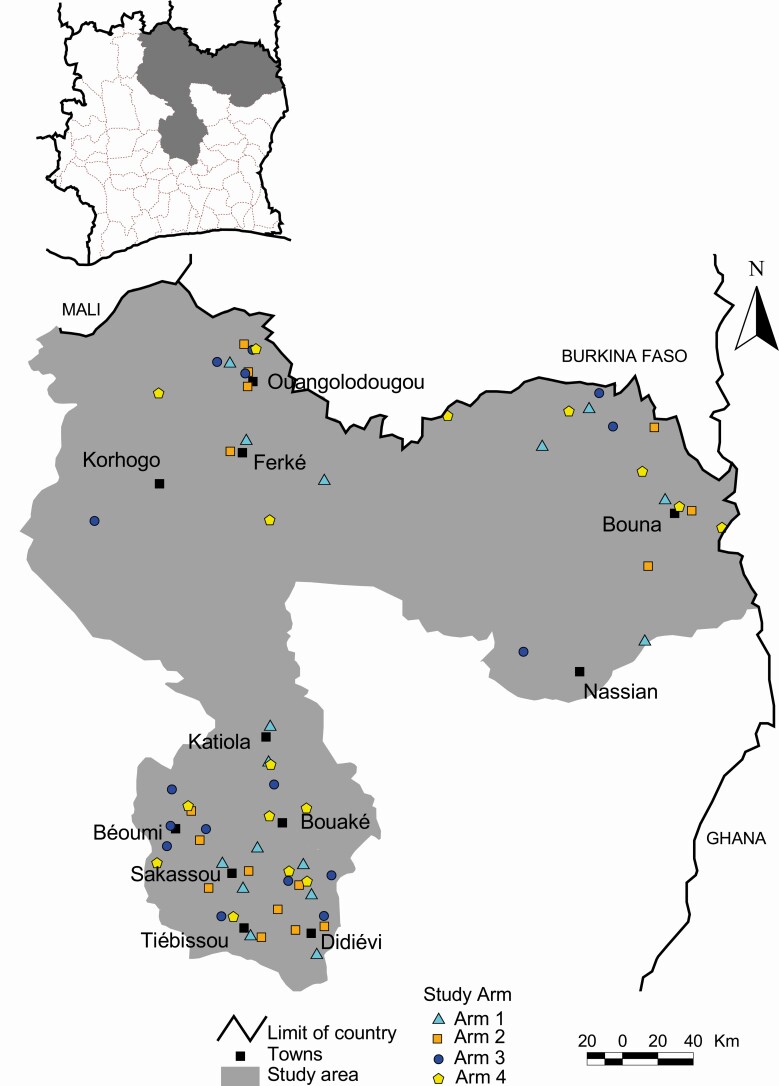
Sixty-four villages of the administrative regions of the northern (Tchologo, Poro, Bounkani, and Hambol) and the central (Gbêkê and Bélier) parts of Côte d’Ivoire were selected. Although the study protocol called for enrollment of 60 villages, 64 villages met enrollment criteria and were included because of promises made during the consenting for the screening survey. The inclusion of the 4 additional villages was approved by funders (SCORE). Thus, there were 16 villages (instead of 15 according to the study protocol) per study arm (Figure 1).
Figure 1.
Map of the study area showing the 64 study villages in northern and central parts of Côte d’Ivoire by intervention arms. Arm 1: Annual MDA with praziquantel before the peak schistosomiasis transmission season. Arm 2: Annual MDA with praziquantel after the peak schistosomiasis transmission season. Arm 3: MDA biannual treatment. Arm 4: Annual MDA with praziquantel before the peak schistosomiasis transmission season plus snails control with niclosamide. Abbreviation: MDA, mass drug administration.
Study Design and Interventions
The study was a 3-year cluster-randomized trial with 4 intervention arms: (1) villages received annual MDA with praziquantel before the peak of schistosomiasis transmission season (this can be considered as the recommended standard of care in this setting and is therefore used as reference arm); (2) annual MDA with praziquantel after the peak of schistosomiasis transmission season; (3) biannual treatments before and after the peak of schistosomiasis transmission season; and (4) annual MDA with praziquantel before the peak of schistosomiasis transmission season, coupled with snail control using niclosamide.
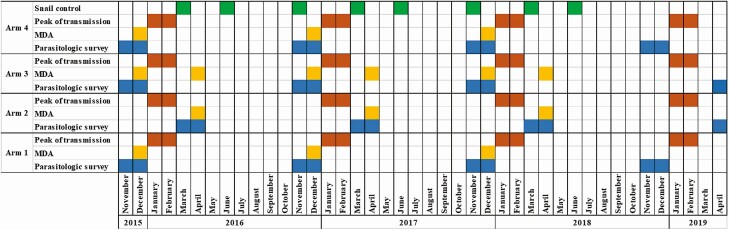
A few days before each MDA, a parasitologic survey was conducted in the study villages among children aged 5–12 years and adults aged 20–55 years, except for the second MDA in arm 3. The final parasitologic data collection was pursued in each village, 1 year after the last MDA by each study arm.
Difference in S. haematobium prevalence and intensity of infection between study arms was assessed in the 3 study groups at the final survey with the primary objective focused on 9- to 12-year-old children.
Parasitologic Survey
In each village, a total of 100 children aged 9–12 years, 50 children aged 5–8 years, and 50 adults aged 20–55 years were randomly selected per survey to participate in parasitologic assessments. After obtaining written informed consent, participants were invited to provide a urine sample, produced between 10:00 and 14:00 hours. Urine samples were examined for macrohematuria, microhematuria, and presence of S. haematobium eggs by visual inspection, reagent strip testing (Haemastix; Siemens Healthcare Diagnostics GmbH, Camberley, Surrey, UK), and urine filtration, respectively.
After the baseline survey conducted in all the study villages, follow-up surveys were done in villages of arms 1, 3, and 4 in November and December each year from 2016 to 2017. Villages of arm 2 were surveyed in March and April of each year from 2017 to 2018, according to the study design. The final parasitologic survey was carried out in November/December 2018 for arms 1 and 4, and April 2019 for arms 2 and 3, 1 year after the final MDA in each study arm (Figure 2).
Figure 2.
Timeline of study activities including parasitologic surveys (blue), MDA (yellow), peak of transmission (orange), and snail control surveys (green). Abbreviation: MDA, mass drug administration.
Snail Control Using Niclosamide
Snail control was implemented only in villages of arm 4 using niclosamide, the main molluscicide recommended by WHO [19, 20]. Malacologic surveys were conducted from 2016 to 2018, 3 times per year, in November, March, and June. Snails collected were identified by genus and, whenever possible, by species level, and densities were assessed at human-water contact sites. During each survey, those human-water contact sites found with Bulinus spp. (B. truncatus and B. globosus; the intermediate hosts of S. haematobium in this part of Côte d’Ivoire) were treated with niclosamide (concentration of 10 g/L) and were revisited the next day to determine whether snails might have survived.
MDA Approaches
All individuals aged 5 years and above in the study villages were eligible for a free-of-charge preventive chemotherapy with a single 40 mg/kg oral dose of praziquantel, according to WHO guidelines. Both school-based treatment (SBT) and community-wide treatment (CWT) approaches were employed to increase the treatment coverage. Pupils were treated at school by trained teachers, whereas children not enrolled at school and adults were treated by trained community health workers who adopted a door-to-door approach.
The annual MDA in arms 1 and 4 and the first treatment in arm 3 occurred each December from 2015 to 2017. The second MDA in arm 3 and the annual treatment in arm 2 were each carried out in April from 2016 to 2018 (Figure 2). The treatment coverage per village was assessed by dividing the number of individuals aged 5 years and above who were treated by the total number of people of the relevant age group. We estimated the total population for each village based on the 2014 census, assuming an annual increase in population of 2.6% based on the estimated national growth rate. We assumed that 84% of the total population of each village would be in the age groups eligible for MDA based on the age distribution in the 2014 census.
Statistical Analysis
Details on the number of clusters and participants and eligibility criteria have been published elsewhere [14]. In brief, data were double entered into an Excel spreadsheet (Microsoft Corporation; Redmond, Washington, USA), and cross-checked using EpiInfo version 3.4 (Centers for Disease Control and Prevention; Atlanta, Georgia, USA). The databases were uploaded and maintained on a central server (Open Data Kit) in Atlanta, Georgia, USA. For prevalence estimates, participants were considered infected if there was at least 1 S. haematobium egg discovered in 10 mL of filtered urine, examined under a microscope. The relative difference (% change) in S. haematobium prevalence between baseline and final surveys was calculated as follows: prevalence reduction rate = {[(prevalence at final survey − prevalence at baseline)/prevalence at baseline] × 100}. The arithmetic mean (AM) of infection intensity was estimated at village-level, expressed as S. haematobium eggs per 10 mL of urine. Infected children were classified as having light infection (1–49 eggs per 10 mL of urine) and heavy infection (≥50 eggs per 10 mL of urine), according to WHO thresholds [21]. S. haematobium egg counts were truncated at 1000 eggs per 10 mL urine. The reduction rate in infection intensity was calculated as follows: [(1 − AM eggs per 10 mL of urine at final survey/AM eggs per 10 mL of urine at baseline) × 100].
The primary analysis estimated differences between study arms in the final survey according to statistical analysis plans developed by SCORE investigators prior to data being available in this study [22, 23]. Differences in prevalence were evaluated using generalized estimating equations (GEE) for binary distributed outcomes with logit link and independent correlation structure to account for potential intra-class correlations within village clusters. Annual MDA before the peak of transmission (arm 1) was designated as the reference group. The secondary analysis used the same model but included baseline prevalence, sex, and age as additional covariates, and the model was weighted according to the number of observations in each village. Differences in egg counts were assessed using GEE for negative binomial distributed outcomes with log link and independent correlation structure. Adjusted and unadjusted models were estimated in the same way as the prevalence models. The primary analysis was done in R version 3.5.4.
We observed some imbalance among trial arms with respect to baseline prevalence. Hence, we decided to run, in addition to the prespecified models, an explorative analysis using inverse probability weighting (IPW) to adjust for baseline imbalances.
RESULTS
Study Flow
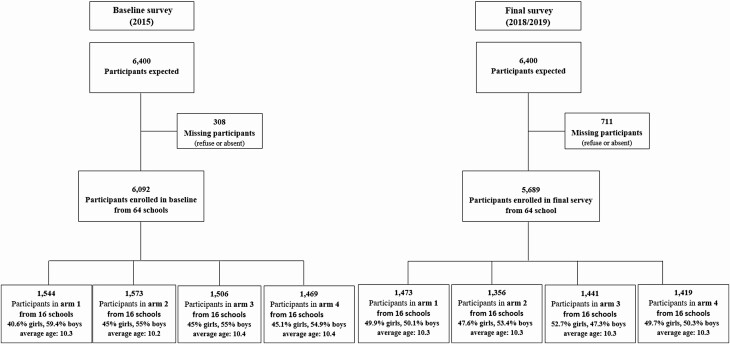
The study, including the eligibility survey, was carried out between May 2015 and May 2019. Overall, 208 villages were subjected to an eligibility survey, of which 64 were selected for the study. No village dropped out during the study. At the parasitologic baseline survey, 6092 children aged 9–12 years were enrolled and 5689 at the final survey (Figure 3).
Figure 3.
Study profile among children aged 9–12 years during baseline and last surveys. Final survey was carried out in November/December 2018 for arms 1 and 4 and in April/May 2019 for arms 2 and 3. Arm 1: Annual MDA with praziquantel before the peak schistosomiasis transmission season. Arm 2: Annual MDA with praziquantel after the peak schistosomiasis transmission season. Arm 3: MDA biannual treatment. Arm 4: Annual MDA with praziquantel before the peak schistosomiasis transmission season plus snails control with niclosamide. Abbreviation: MDA, mass drug administration.
In addition, 3138 children aged 5–8 years were enrolled in the baseline and 3028 in the final survey. As regards adults aged 20–55 years, there were 3007 in the baseline and 2394 in the endline survey.
Differences in Prevalence and Intensity of S. haematobium Infection
At the baseline survey, the prevalence of S. haematobium in children aged 9–12 years was 24.8% in arm 1, 10.1% in arm 2, 13.9% in arm 3, and 15.9% in arm 4. The AM egg counts per 10 mL of urine ranged from 5.7 eggs to 17.9 eggs between arms (Table 1). At the final survey, the prevalence and the AM egg count decreased in all study arms.
Table 1.
Descriptive Results for Baseline and Final Survey per Study Arm (All Study Age Groups)
| Children Aged 9–12 Years | Children Aged 5–8 Years | Adults Aged 20–55 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 1 | Arm 2 | Arm 3 | Arm 4 |
| Number of villages | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Number of people tested at baseline | 1544 | 1573 | 1506 | 1469 | 820 | 788 | 804 | 726 | 727 | 761 | 762 | 759 |
| Proportion female N (%) at baseline | 627 (40.6) | 708 (45.0) | 672 (44.6) | 662 (45.0) | 427 (52.1) | 383 (48.6) | 378 (47.0) | 342 (47.1) | 434 (59.7) | 473 (62.1) | 461 (60.5) | 443 (58.4) |
| Number of people infected at baseline | 383 | 159 | 210 | 233 | 147 | 50 | 87 | 48 | 85 | 46 | 70 | 85 |
| Prevalence at baseline (%) | 24.8 | 10.1 | 13.9 | 15.9 | 17.9 | 6.4 | 10.8 | 6.6 | 11.7 | 6.0 | 9.2 | 11.0 |
| Number of villages | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Number of people tested in final survey | 1473 | 1441 | 1419 | 1356 | 812 | 771 | 755 | 789 | 646 | 611 | 598 | 593 |
| Proportion female N (%) in final survey | 735 (49.9) | 693 (48.1) | 728 (51.3) | 677 (49.9) | 414 (51.0) | 382 (49.5) | 385 (51.0) | 385 (48.8) | 352 (54.5) | 356 (58.3) | 337 (56.3) | 312 (52.6) |
| Number of people infected in final survey | 111 | 50 | 8 | 46 | 66 | 27 | 19 | 22 | 11 | 14 | 11 | 3 |
| Prevalence at final survey (%) | 7.5 | 3.5 | 0.6 | 3.4 | 8.1 | 3.5 | 2.5 | 2.8 | 1.7 | 2.3 | 1.8 | 0.5 |
| Absolute difference between prevalence at final survey and baseline | −17.3 | −6.6 | −13.4 | −12.5 | −9.8 | −2.9 | −8.3 | −3.8 | −10.0 | −3.8 | −7.3 | −10.7 |
| Relative difference in prevalence between final survey and baseline (% change) | −69.6 | −65.7 | −96.0 | −78.6 | −54.7 | −44.9 | −76.7 | −57.8 | −85.5 | −62.1 | −80.0 | −95.5 |
| Village-level arithmetic mean infection intensity at baseline (including zeros) | 17.9 | 5.7 | 8.4 | 6.2 | 9.6 | 4.1 | 4.5 | 2.0 | 11.7 | 6.0 | 9.2 | 11.0 |
| Village-level arithmetic mean infection intensity at final survey (including zeros) | 11.7 | 1.6 | 0.3 | 0.7 | 8.2 | 3.2 | 0.5 | 0.6 | 0.4 | 0.3 | 0.3 | 0.1 |
| Egg reduction rate (%) | 34.6 | 71.9 | 96.4 | 88.7 | 14.6 | 22.0 | 88.9 | 70.0 | 96.6 | 95.0 | 96.7 | 99.1 |
Arm 1: Praziquantel annual mass drug administration (MDA) before peak of transmission. Arm 2: Praziquantel annual MDA after peak of transmission. Arm 3: Praziquantel MDA biannual treatment. Arm 4: Praziquantel annual MDA before peak of transmission plus snail control. N: case number.
At the final survey, the GEE model revealed that the difference in S. haematobium prevalence was significant between arms 1 and 3 (0.6% vs 7.5%; odds ratio [OR] = 0.07, 95% confidence interval [CI] = .02–.24). The observed prevalence in arms 2 and 4 were 3.5% and 3.4%, respectively, which were not statistically significantly different from arm 1. However, a significant difference was observed in egg counts between arm 1 and the other study arms. Adjusting for baseline imbalance with either covariate adjustment or IPW (Supplementary Appendix) did not change the interpretation of the results (Table 2).
Table 2.
Differences in Prevalence and Intensity of S. haematobium Infection Between Study Arms at the Final Survey
| Prevalence | Intensity | ||||
|---|---|---|---|---|---|
| Age Group | Arms Compared | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted CR (95% CI) | Adjusted CR (95% CI) |
| 9- to 12-year-old | Arm 2 vs Arm 1 | 0.44 (.10–1.92) | 1.67 (.41–6.88) | 0.08 (.01–.66) | 0.38 (.04–3.70) |
| Arm 3 vs Arm 1 | 0.07 (.02–.24) | 0.08 (.02–.33) | 0.01 (.00–.08) | 0.03 (.00–.25) | |
| Arm 4 vs Arm 1 | 0.43 (.11–1.74) | 0.66 (.17–2.53) | 0.04 (.01–.30) | 0.1 (.01–.79) | |
| 5- to 8-year-old | Arm 2 vs Arm 1 | 0.41 (.08–2.13) | 0.64 (.09–4.46) | 0.25 (.02–2.68) | 0.67 (.06–7.43) |
| Arm 3 vs Arm 1 | 0.29 (.07–1.30) | 0.34 (.08–1.56) | 0.06 (.01–.41) | 0.07 (.01–.51) | |
| Arm 4 vs Arm 1 | 0.23 (.04–1.23) | 0.32 (.05–1.98) | 0.03 (.00–.24) | 0.03 (.00–.26) | |
| 20- to 55-year-old | Arm 2 vs Arm 1 | 1.35 (.43–4.29) | 1.91 (.49–7.53) | 0.73 (.18–3.01) | 0.91 (.18–4.56) |
| Arm 3 vs Arm 1 | 1.08 (.32–3.69) | 1.27 (.37–4.41) | 1.02 (.15–6.77) | 0.77 (.10–5.86) | |
| Arm 4 vs Arm 1 | 0.29 (.08–1.02) | 0.34 (.10–1.11) | 0.37 (.04–3.18) | 0.51 (.05–4.64) | |
The model was adjusted for baseline prevalence, sex, and age as additional covariates, and weighted according to the number of observations in each village. Arm 1: Praziquantel annual MDA before peak of transmission. Arm 2: Praziquantel annual MDA after peak of transmission. Arm 3: Praziquantel MDA biannual treatment. Arm 4: Praziquantel annual MDA before peak of transmission plus snail control.
Abbreviations: CI, confidence interval; CR, count ratio; MDA, mass drug administration; OR, odds ratio.
Among first-grade children and adults, no significant difference was observed in prevalence between study arms at the final survey (Table 2).
None of the study arms reached the goal of zero cases of S. haematobium infection (interruption of transmission) at the study end. However, the proportion of villages with zero cases of infection among children aged 9–12 years increased to >40% within all study arms between the baseline and the final surveys (Table 3).
Table 3.
Proportion of Villages that Reached Incidence 0 New Cases of S. haematobium Infection and those that Reached Elimination as a Public Health Problem From Baseline to Final Survey per Study Arm (All the Study Age Groups Combined)
| Children Aged 9–12 Years | All Age Groups | ||||
|---|---|---|---|---|---|
| Study Year | Arm | Villages with Zero Cases N (%) | Villages EPHP N (%) | Villages with Zero Cases n (%) | Villages EPHP N (%) |
| Baseline | 1 | 0 (0.0) | 4 (25.0) | 0 (0.0) | 5 (31.3) |
| 2 | 2 (12.5) | 10 (62.3) | 0 (0.0) | 10 (62.3) | |
| 3 | 2 (12.5) | 8 (50.0) | 1 (6.3) | 9 (56.3) | |
| 4 | 2 (12.5) | 7 (43.8) | 0 (0.0) | 6 (37.5) | |
| Follow-up 1 | 1 | 5 (31.3) | 12 (75.0) | 3 (18.8) | 12 (75.0) |
| 2 | 9 (56.3) | 14 (87.5) | 5 (31.3) | 15 (93.8) | |
| 3 | 10 (62.5) | 16 (100) | 6 (37.5) | 16 (100) | |
| 4 | 10 (62.5) | 13 (81.3) | 7 (43.8) | 14 (87.5) | |
| Follow-up 2 | 1 | 7 (43.3) | 11 (68.8) | 4 (25.0) | 13 (81.3) |
| 2 | 10 (62.5) | 15 (93.8) | 7 (43.3) | 15 (93.8) | |
| 3 | 9 (56.3) | 15 (93.8) | 4 (25.0) | 16 (100) | |
| 4 | 9 (56.3) | 12 (75.0) | 5 (31.3) | 14 (87.5) | |
| Final survey | 1 | 9 (56.3) | 12 (75.0) | 7 (43.3) | 13 (81.3) |
| 2 | 7 (43.3) | 11 (68.8) | 4 (25.0) | 13 (81.3) | |
| 3 | 9 (56.3) | 15 (93.8) | 5 (31.3) | 16 (100) | |
| 4 | 8 (50.0) | 14 (87.5) | 7 (43.3) | 14 (87.5) | |
Arm 1: Praziquantel annual MDA before peak of transmission. Arm 2: Praziquantel annual MDA after peak of transmission. Arm 3: Praziquantel MDA biannual treatment. Arm 4: Praziquantel annual MDA before peak of transmission plus snail control.
Abbreviations: EPHP, elimination as a public health problem (heavy infection <1%); MDA, mass drug administration; N, case number.
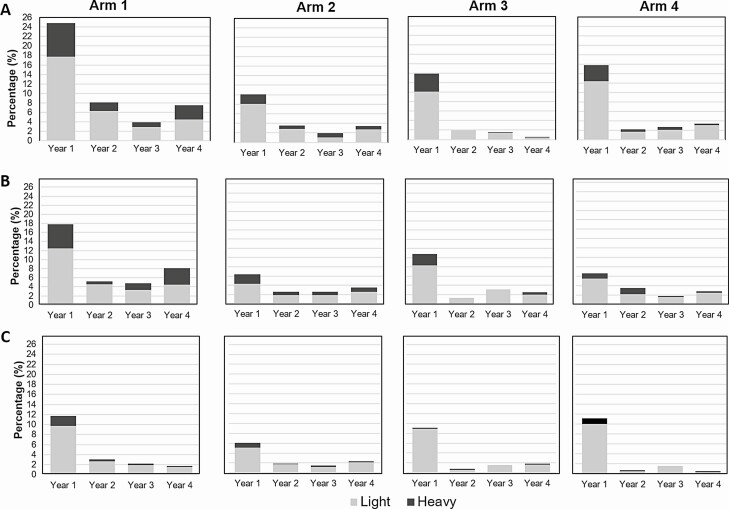
Heavy intensity infections decreased from baseline to final survey among all age groups in all arms (Figure 4). Proportion of villages with heavy infections <1% in final survey increased compared to baseline in all study arm (Table 3). However, 1 village alone recorded 36 of the 45 cases of heavy infection in arm 1 at the final survey.
Figure 4.
Overall prevalence stratified by infection intensity category by arm and by study year among (A) children aged 9–12 years, (B) children aged 5–8 years, and (C) adults aged 20–55 years. Total bar height represents S. haematobium infection prevalence in each year. Gray represents the prevalence of individuals with light intensity infections (1–49 eggs/10 mL of urine); and black represents prevalence of individuals with heavy intensity infections (≥50 eggs/10 mL of urine). Arm 1: Annual MDA with praziquantel before the peak schistosomiasis transmission season. Arm 2: Annual MDA with praziquantel after the peak schistosomiasis transmission season. Arm 3: MDA biannual treatment. Arm 4: Annual MDA with praziquantel before the peak schistosomiasis transmission season + snails control with niclosamide. Abbreviation: MDA, mass drug administration.
Snail Control With Niclosamide
The malacologic surveys made in June recorded the highest number of human-water contact sites visited per year. Both intermediate host species (ie, B. truncatus and B. globosus) were present, although B. truncatus was the predominant species. The percentage of human-water contact sites found with these snails varied between 7.4% and 33.3%, and all were treated with niclosamide during the months indicated (Table 4). The day after each treatment, the snails found in the human-water contact sites were all dead.
Table 4.
Snail Control Coverage in the 16 Villages of Arm 4 Over Intervention Period With Niclosamide
| Malacologic Survey Period | Human-Water Contacts sites | Human-Water Contact Sites with B. truncatus (%) | Human Water Contact Sites with B. globosus (%) | Total Bulinus spp. | Human-Water Contact Sites Treated with Niclosamide (%) |
|---|---|---|---|---|---|
| March 2016 | 23 | 5 (21.7) | 0 (0) | 837 | 5 (21.7) |
| June 2016 | 55 | 5 (9.1) | 1 (1.8) | 649 | 6 (10.9) |
| November 2016 | 47 | 6 (12.8) | 3 (6.4) | 329 | 9 (19.1) |
| March 2017 | 39 | 6 (15.4) | 3 (7.7) | 1201 | 9 (23.1) |
| June 2017 | 54 | 4 (7.4) | 0 (0) | 545 | 4 (7.4) |
| November 2017 | 41 | 6 (14.6) | 4 (9.8) | 1242 | 10 (24.4) |
| March 2018 | 27 | 6 (22.2) | 3 (11.1) | 754 | 9 (33.3) |
| June 2018 | 39 | 4 (10.2) | 3 (7.7) | 355 | 7 (17.9) |
MDA Coverage
MDA treatment coverage varied between study villages and from one treatment round to another. Table 5 shows MDA coverage by study arm over the course of the trial. The lowest coverage was observed in arm 4, varying from 65.6% in the first MDA round to 79.4% in the final round.
Table 5.
Coverage of Mass Drug Administration (MDA) in Study Arm Over the 3-Year Intervention Period
| Study Arm | Population | Treatment Period | |||||
|---|---|---|---|---|---|---|---|
| December 2015 | April 2016 | December 2016 | April 2017 | December 2017 | April 2018 | ||
| 1 | Target population | 17 226 | … | 17 657 | … | 18 099 | … |
| Treated population | 13 930 | … | 15 168 | … | 18 347 | … | |
| Coverage (%) | 80.9 | 85.9 | 101.4 | ||||
| 2 | Target population | … | 21 381 | … | 21 916 | … | 22 464 |
| Treated population | … | 16 391 | … | 18 325 | … | 14 401 | |
| Coverage (%) | 76.7 | 83.6 | 64.1 | ||||
| 3 | Target population | 27 707 | 28 418 | 28 418 | 29 112 | 29 112 | 29 844 |
| Treated population | 19 757 | 23 849 | 22 710 | 22 539 | 25 615 | 20 162 | |
| Coverage (%) | 71.3 | 83.9 | 79.9 | 77.4 | 88.0 | 67.6 | |
| 4 | Target population | 27 031 | … | 27 707 | … | 28 399 | … |
| Treated population | 17 718 | … | 18 757 | … | 22 543 | … | |
| Coverage (%) | 65.6 | 67.7 | 79.4 | ||||
DISCUSSION
The WHO Strategic Plan 2012–2020 [6] called upon member states to attempt elimination of schistosomiasis as a public health problem and to interrupt transmission in selected areas by 2025. We implemented this cluster-randomized trial to assess 4 schedules to interrupt S. haematobium transmission in the northern and central parts of Côte d’Ivoire, characterized by seasonal transmission (transmission of schistosomiasis is not continuous throughout the year but rather linked to the season). In these settings, optimally timed drug administration and other interventions could increase the impact of schistosomiasis control and interruption of transmission seems possible. In line with WHO recommendations for interruption of transmission, MDA in our study was extended to all age groups. However, for the assessments, 9 to 12-year-old children were particularly targeted, as this age group is at high risk of schistosomiasis and sample collection is relatively straightforward.
MDA in schistosomiasis is advised during the dry season [24], but peak of transmission as a benchmark received little attention thus far. Our results showed that the timing of MDA (before or after peak transmission) had no effect on transmission. Previous studies concluded that the timing of MDA had a less pronounced effect than that of snails control [25]. We found a significant difference in S. haematobium prevalence at the final survey when comparing the arm with biannual treatment (arm 3) with the reference arm with annual MDA before peak transmission season (arm 1). A likely explanation of this observation is that more frequent treatment exerts pressure on interrupting transmission. Layering intermediate host snail control on top of annual MDA did not achieve a significant difference in reducing the prevalence and intensity of S. haematobium infection, as compared to the reference arm. This observation might be explained by the complexity of snail control, linked sometimes to the water surface size to be treated with niclosamide or to the difficulty to identify all human-water contact sites. In addition, niclosamide application is focal and sporadic, and hence, does not prevent the repopulation of treated areas by intermediate host snails. Our results corroborate findings obtained from a previous SCORE study in Zanzibar, Tanzania [26].
No study arm achieved the goal of zero cases of S. haematobium infection at the study end. Ongoing transmission in the study area might be explained by people who missed MDA, preschool-age children who have not been considered in treatments with praziquantel thus far, or in-migrating people infected with S. haematobium. It should be noted that some villages went from zero cases 1 year to new cases and even heavy infections the following year. These observations underscore the need to extend interventions for elimination to a longer time frame than in the current study and perhaps using additional control measures [27, 28]
Importantly, though, the secondary objective of eliminating S. haematobium as a public health problem was achieved in most of the study villages. However, there was a “persistent hotspot” [29, 30], where 36 cases of heavy intensity infections were clustered at the final survey. As already indicated by others, treatment alone fails to interrupt transmission in such contexts [31].
Beyond the limit of snail control articulated before, another limitation of our study is the underestimation of the true prevalence and infection intensity, because of the diagnostic approach used in the current trial. Indeed, there is considerable day-to-day variation of S. haematobium egg excretion and low-intensity infections are likely to be missed when only single urine samples are being processed in the laboratory [32, 33].
CONCLUSION
All 4 intervention regimens investigated substantially reduced the prevalence and intensity of S. haematobium infection. The study arm with biannual MDA was the only approach tested that showed a significantly greater reduction in the prevalence and intensity of infection at the end of trial when compared to standard annual MDA scheduling. None of study interventions achieved the interruption of S. haematobium transmission. However, proportion of villages with zero cases of infection substantially increased at the study end within all study arms and most of them reached the goal of S. haematobium elimination as a public health problem. Snail control did not significantly improve the effect of MDA in our study. However, we recommend assessment of snail control combined with a more intensive MDA program for a longer period to better appreciate a potential impact on transmission interruption.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the SCORE secretariat (Daniel G. Colley, Carl H. Campbell Jr, Charles H. King, and Sue Binder), and Abdoulaye Meïté and his team from the “Programme National de Lutte contre les Maladies Tropicales Négligées à Chimiothérapie Préventive” in Côte d’Ivoire. They thank the laboratory technicians who contributed to this work and all the teachers and village leaders in the project areas for their contribution to population sensitization.
Authors’ contributions. M. O., J. H., J. T. C., J. U., and E. K. N. designed the study protocol according to SCORE guidelines. M. O., F. K. B., N. R. D., Y.-N. T. T.-B., C. K. K., R. K. A., N. K., and N. G.-C. collected data. J. H. and P. K. Y. assisted with data management and statistical analysis. M. O. wrote the first draft of the manuscript. All authors contributed to the manuscript revision and approved the final version prior to submission.
Financial support. This study was supported by the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) through a grant provided to the University of Georgia Research Foundation, Inc. from the Bill & Melinda Gates Foundation (grant numbers: prime award number 50816; subaward number RR374-053/4787986). Drugs were provided by the Schistosomiasis Control Initiative (SCI) based at Imperial College London, UK.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Mamadou Ouattara, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
Fidèle K Bassa, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
Nana R Diakité, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
Jan Hattendorf, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Jean T Coulibaly, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire; Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Patrick K Yao, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire.
Yves-Nathan T Tian-Bi, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
Cyrille K Konan, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
Rufin K Assaré, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
Naférima Koné, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire.
Négnorogo Guindo-Coulibaly, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire.
Jürg Utzinger, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Eliézer K N’Goran, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d’Ivoire; Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire.
References
- 1. WHO. Schistosomiasis: number of people receiving preventive chemotherapy in 2012. Wkly Epidemiol Rec 2014; 89:21–8. [PubMed] [Google Scholar]
- 2. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014; 383:2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organ Tech Rep Ser 2002; 912:1–57. [PubMed] [Google Scholar]
- 4. WHO. World Health Assembly Resolution WHA 54.19. Schistosomiasis and soil-transmitted helminth infections. 2001. Available at: www.who.int/neglected_diseases/mediacentre/WHA_54.19_Eng. Accessed 17 June 2020.
- 5. WHO. A roadmap for implementation: accelerating work to overcome the global impact of neglected tropical diseases. Geneva: World Health Organization, 2012. Available at: www.who.int/iris/bitstream/handle/10665/70809/WHO_HTM_NTD_2012.1_eng. Accessed 15 June 2020. [Google Scholar]
- 6. WHO. Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. Geneva: World Health Organization, 2013. [Google Scholar]
- 7. WHO. World Health Assembly Resolution WHA 65.21. Elimination of schistosomiasis. Geneva: World Health Organization, 2012. Available at: https://www.who.int/neglected_diseases/mediacentre/WHA_65.21_Eng. Accessed 17 June 2020. [Google Scholar]
- 8. King CH, Sutherland LJ, Bertsch D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl Trop Dis 2015; 9:e0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sokolow SH, Wood CL, Jones IJ, et al. . Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis 2016; 10:e0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo NC, Addiss DG, Hotez PJ, et al. . A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect Dis 2017; 17:e64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ezeamama AE, He CL, Shen Y, et al. . Gaining and sustaining schistosomiasis control: study protocol and baseline data prior to different treatment strategies in five African countries. BMC Infect Dis 2016; 16:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colley DG. Morbidity control of schistosomiasis by mass drug administration: how can we do it best and what will it take to move on to elimination? Trop Med Health 2014; 42:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ouattara M, Diakité NR, Yao PK, et al. . Effectiveness of school-based preventive chemotherapy strategies for sustaining the control of schistosomiasis in Côte d’Ivoire: results of a 5-year cluster randomized trial. PLoS Negl Trop Dis 2021; 15:e0008845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian-Bi YT, Ouattara M, Knopp S, et al. . Interrupting seasonal transmission of Schistosoma haematobium and control of soil-transmitted helminthiasis in northern and central Côte d’Ivoire: a SCORE study protocol. BMC Public Health 2018; 18:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diakité NR, Ouattara M, Bassa FK, et al. . Baseline and impact of first-year intervention on Schistosoma haematobium infection in seasonal transmission foci in the northern and central parts of Côte d’Ivoire. Trop Med Infect Dis 2021; 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yapi RB, Hürlimann E, Houngbedji CA, et al. . Infection and co-infection with helminths and Plasmodium among school children in Côte d’Ivoire: results from a national cross-sectional survey. PLoS Negl Trop Dis 2014; 8:e2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krauth SJ, Greter H, Stete K, et al. . All that is blood is not schistosomiasis: experiences with reagent strip testing for urogenital schistosomiasis with special consideration to very-low prevalence settings. Parasit Vectors 2015; 8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schur N, Hürlimann E, Garba A, et al. . Geostatistical model-based estimates of schistosomiasis prevalence among individuals aged ≤20 years in West Africa. PLoS Negl Trop Dis 2015; 5:e1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO. The WHO recommended classification of pesticides by hazard and guidelines to classification 2009. 2010. Available at: www.who.int/iris/bitstream/handle/10665/44271/9789241547963_eng. Accessed 10 June 2020.
- 20. WHO. Field use of molluscicides in schistosomiasis control programmes: an operational manual for programme managers. Geneva: World Health Organization, 2017. [Google Scholar]
- 21. Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L.. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization, 1998. [Google Scholar]
- 22. Campbell CH, Binder S, King CH, et al. . SCORE operational research on moving toward interruption of schistosomiasis transmission. Am J Trop Med Hyg 2020; 103(1-suppl):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King CH, Kittur N, Binder S, et al. . Impact of different mass drug administration strategies for gaining and sustaining control of Schistosoma mansoni and Schistosoma haematobium infection in Africa. Am J Trop Med Hyg 2020; 103(1-suppl):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gbalégba NGC, Silué KD, Ba O, et al. . Prevalence and seasonal transmission of Schistosoma haematobium infection among school-aged children in Kaedi town, southern Mauritania. Parasit Vectors 2017; 10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Q, Gurarie D, Ndeffo-Mbah M, Li E, King CH. Schistosoma transmission in a dynamic seasonal environment and its impact on the effectiveness of disease control. J Infect Dis 2020; jiaa746. Available at: 10.1093/infdis/jiaa746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knopp S, Person B, Ame SM, et al. . Evaluation of integrated interventions layered on mass drug administration for urogenital schistosomiasis elimination: a cluster-randomised trial. Lancet Glob Health 2019; 7:e1118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson RM, Turner HC, Farrell SH, Yang J, Truscott JE. What is required in terms of mass drug administration to interrupt the transmission of schistosome parasites in regions of endemic infection? Parasit Vectors 2015; 8:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kajihara N, Hirayama K. The war against a regional disease in Japan: a history of the eradication of Schistosomiasis japonica. Trop Med Health 2011; 39:3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiegand RE, Mwinzi PNM, Montgomery SP, et al. . A persistent hotspot of Schistosoma mansoni infection in a five-year randomized trial of praziquantel preventative chemotherapy strategies. J Infect Dis 2017; 216:1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kittur N, Binder S, Campbell CH, et al. . Defining persistent hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with praziquantel for control of schistosomiasis. Am J Trop Med Hyg 2017; 97:1810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. King CH. Toward the elimination of schistosomiasis. N Engl J Med 2009; 360:106–9. [DOI] [PubMed] [Google Scholar]
- 32. Colley DG, Andros TS, Campbell CH Jr. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty 2017; 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amoah AS, Hoekstra PT, Casacuberta-Partal M, et al. . Sensitive diagnostic tools and targeted drug administration strategies are needed to eliminate schistosomiasis. Lancet Infect Dis 2020; 20:e165–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.