Abstract
Electrical cortical stimulation is widely performed and is the gold standard for functional mapping in intractable epilepsy patients; however, a standard protocol has not yet been established. With respect to stimulation methods, two techniques can be applied: monopolar and bipolar stimulation. We compared the threshold to induce clinical symptoms between these two stimulation techniques. Twenty patients with intractable epilepsy who underwent electrical cortical stimulation for functional mapping were retrospectively investigated. We evaluated the stimulation intensity thresholds required to induce motor, sensory, and language symptoms. A total of 114 electrodes in 20 patients were used to investigate motor, sensory, and language symptoms. The thresholds required to induce motor (median value, bipolar: 4 mA, monopolar: 5 mA, p < 0.05) and language symptoms (bipolar: 8 mA, monopolar: 10 mA, p < 0.0005) were significantly higher for monopolar stimulation than those for bipolar stimulation. However, for sensory symptoms, no significant differences were found in the required thresholds between monopolar and bipolar stimulation (bipolar: 4 mA, monopolar: 4 mA, p = 0.474). Bipolar cortical stimulation required lower intensities to produce clinical motor and language symptoms and thus would be safe and suitable for screening of the eloquent area in functional mapping.
Keywords: functional brain mapping, electrical cortical stimulation, bipolar stimulation, monopolar stimulation, epilepsy surgery
Introduction
Electrical cortical stimulation is widely performed for functional mapping in patients with medically intractable epilepsy. Although this technique is the gold standard for identification of eloquent areas, this technique is not standardized across centers.1,2) The main purpose of electrical cortical stimulation is to identify eloquent cortical regions and preserve neurological function during resection surgery. Electrical cortical stimulation can be performed in two ways: bipolar and monopolar stimulation.1-3) Bipolar stimulation uses adjacent pairs of electrodes, while monopolar stimulation uses a single electrode in reference to a distant electrode overlying the non-eloquent cortex. While some studies have described the difference between these two stimulation methods,4,5) their advantages and disadvantages have not yet been established.
In this study, we compared the threshold of these two stimulation methods to induce clinical symptoms in order to elucidate each characteristic and establish the most beneficial procedure for electrical cortical stimulation-based functional mapping.
Materials and Methods
1. Patients
We analyzed data from 20 patients (11 males, 9 females, median age: 20 years old, range: 8-43 years old) with medically intractable epilepsy who underwent 50-Hz electrical cortical stimulation for functional mapping between October 2016 and May 2021 in Sapporo Medical University Hospital. Written informed consent was obtained from all the patients, and the study protocol was approved by the Institutional Review Board Committee of our institution (No. 23-165).
2. Implantation of electrodes
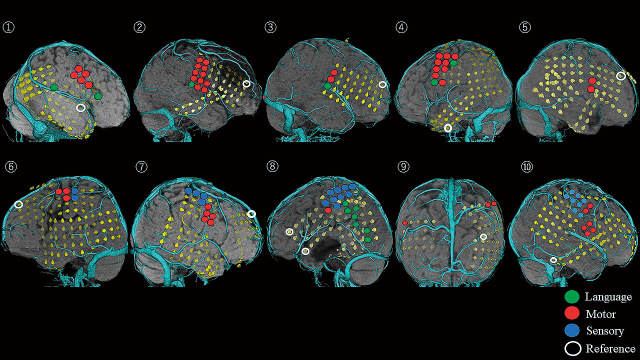
In all patients, strip or grid subdural electrodes (Unique Medical Co., Ltd., Tokyo, Japan) were implanted in the lateral, mesial, and basal aspects of each hemisphere. The additional strip electrodes were placed on the skulls for neutral, reference, and system reference electrodes. The grids consisted of 2 or 4 lines with each line containing 5-8 platinum electrodes and a center-to-center interelectrode distance of 10 mm. The electrodes were made of platinum with a recording diameter of 3 mm. The strip consisted of single lines of 4-6 electrodes in the same configuration as that used for the grids. The locations of the implanted electrodes were confirmed using pre-surgical 3-dimensionally reconstructed magnetic resonance imaging coordinated with postoperative high-resolution volumetric computed tomography (1 mm thin slice) to supply a visual correlation between the location of each electrode and the corresponding cortical area or deep structure (Figs. 1 and 2).
Fig. 1.
The locations of electrodes and eloquent cortices in Pt. 1-10.
The green, red, and blue circles indicate the electrodes that elicited the language, motor, and sensory symptoms in the functional mapping, respectively. The white circles indicate the reference electrodes in monopolar cortical stimulation.
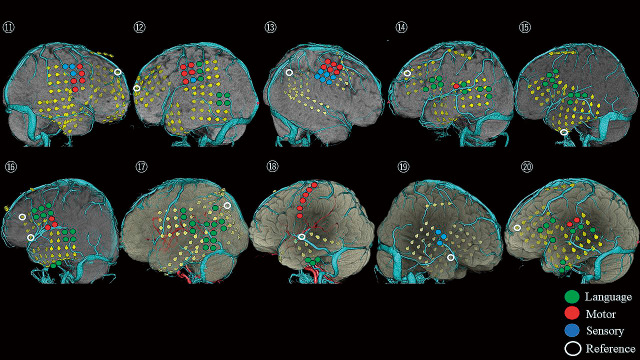
Fig. 2.
The locations of electrodes and eloquent cortices in Pt. 11-20.
3. Electrical cortical stimulation
Repetitive square wave electrical currents of alternating polarity with a pulse width of 0.3 ms were delivered at a frequency of 50 Hz for 5 s. The electric current was increased from 0 to −15 mA in 1 mA steps until a clinical response was observed. In all trials, the stimulation was performed at least twice to check for reproducibility. All electrodes were screened in bipolar mode; thereafter, the eloquent pair was stimulated in monopolar mode (Fig. 3). The symptoms elicited by the stimulation and intensity thresholds for these responses were evaluated. Electrocorticography was recorded to eliminate the effect of afterdischarges.
Fig. 3.
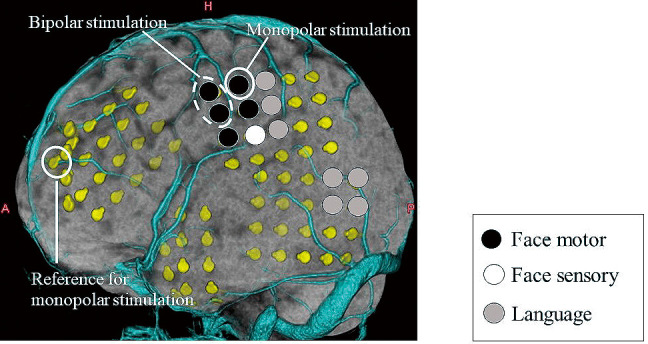
Bipolar and monopolar stimulation.
Bipolar stimulation uses adjacent pairs of electrodes, while monopolar stimulation uses a single electrode in reference to a distant electrode overlying the non-eloquent cortex.
4. Evaluation of clinical signs
We evaluated three clinical signs of language, motor, and sensory symptoms. Language areas were defined where the stimulation elicited an interruption in sentence reading, speech production, and verbal comprehension in the absence of positive and negative tongue motor responses during language tasks (sentence reading, spontaneous speech, object naming, and verbal command). Motor and sensory areas were identified by positive motor responses (muscle twitch) and subjective sensory sensations, respectively.
We evaluated the locations and intensity thresholds to induce these clinical signs by stimulating each cortical region.
5. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 26 (IBM Corp., Armonk, New York, USA). Values were statistically compared between bipolar and monopolar stimulation. Data for each endpoint were reported as medians (interquartile range: IQR, 25th to 75th percentile) and evaluated by the Wilcoxon signed-rank test. p < 0.05 was considered to be statistically significant.
Results
Three clinical signs of language, motor, or sensory symptoms were observed using 114 electrodes in 20 patients during extraoperative electrical cortical stimulation. Table 1 shows the median and range of stimulus thresholds for each clinical symptom in 20 patients.
Table 1.
Stimulation intensity threshold required to produce a clinical response for bipolar and monopolar stimulation
| Pt. | Age | Sex | Threshold (mA)
Median (range) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Language | Motor | Sensory | |||||||||
| N | Bipolar | Monopolar | N | Bipolar | Monopolar | N | Bipolar | Monopolar | |||
| 1 | 23 | Male | 2 | 8 | 11 (10-12) | 3 | 4 (2-4) | 4 (4-5) | 0 | NA | NA |
| 2 | 19 | Male | 0 | NA | NA | 8 | 5.5 (3-10) | 6 (4-10) | 0 | NA | NA |
| 3 | 11 | Male | 0 | NA | NA | 3 | 3 (3-4) | 3 (3-4) | 0 | NA | NA |
| 4 | 9 | Male | 0 | NA | NA | 5 | 4 (2-6) | 6 (3-15) | 1 | 6 | 6 |
| 5 | 8 | Female | 0 | NA | NA | 1 | 7 | 9 | 0 | NA | NA |
| 6 | 14 | Female | 0 | NA | NA | 2 | 4.5 (3-6) | 4.5 (3-6) | 1 | 10 | 6 |
| 7 | 19 | Male | 0 | NA | NA | 2 | 4 | 6.5 (5-8) | 3 | 6 (4-6) | 7 (3.5-8) |
| 8 | 20 | Female | 5 | 6 (6-12) | 10 (6-12) | 0 | NA | NA | 2 | 3 | 3 |
| 9 | 8 | Female | 2 | 14 (13-15) | 13 (12-14) | 3 | 6 (2-7) | 4 (2-5) | 0 | NA | NA |
| 10 | 13 | Female | 0 | NA | NA | 3 | 4 (3-8) | 8 (3-10) | 6 | 5 (2-8) | 4 (2-14) |
| 11 | 33 | Male | 0 | NA | NA | 3 | 2.5 (2.5) | 3 (2-3) | 1 | 2 | 2 |
| 12 | 17 | Female | 4 | 8.5 (6-14) | 13.5 (6-15) | 3 | 6 (5-9) | 7 (6-12) | 0 | NA | NA |
| 13 | 25 | Male | 0 | NA | NA | 4 | 5.5 (2-8) | 1.5 (1.5-15) | 3 | 4 (2-8) | 4 (3-7) |
| 14 | 43 | Male | 7 | 12 (7-14) | 10 (7-15) | 1 | 5 | 5 | 0 | NA | NA |
| 15 | 24 | Male | 5 | 6 (6-10) | 10 (8-14) | 0 | NA | NA | 0 | NA | NA |
| 16 | 29 | Male | 6 | 7.5 (6-14) | 8 (8-12) | 2 | 12 (9-15) | 10 (8-12) | 0 | NA | NA |
| 17 | 20 | Female | 7 | 8 (8-12) | 10 (8-14) | 0 | NA | NA | 0 | NA | NA |
| 18 | 23 | Female | 3 | 8 | 8 (8-14) | 0 | NA | NA | 0 | NA | NA |
| 19 | 42 | Male | 0 | NA | NA | 0 | NA | NA | 2 | 7.5 (4-11) | 6 (3-9) |
| 20 | 23 | Female | 9 | 6 (5-10) | 8 (6-13) | 2 | 6 | 6.5 (6-7) | 0 | NA | NA |
Positive motor symptoms were induced by 45 electrodes in 14 patients. Of these electrodes, bipolar stimulation showed lower thresholds than monopolar stimulation in 19 electrodes (42%), comparable thresholds in 18 electrodes (40%), and higher thresholds in 8 electrodes (18%). The median stimulation intensity threshold to produce motor symptoms was 4 mA (IQR: 3-6 mA) for bipolar stimulation and 5 mA (IQR: 3−7 mA) for monopolar stimulation. Statistical analyses revealed that the threshold for monopolar stimulation was significantly higher than that for bipolar stimulation (N = 45, p < 0.05) (Fig. 4a).
Fig. 4.
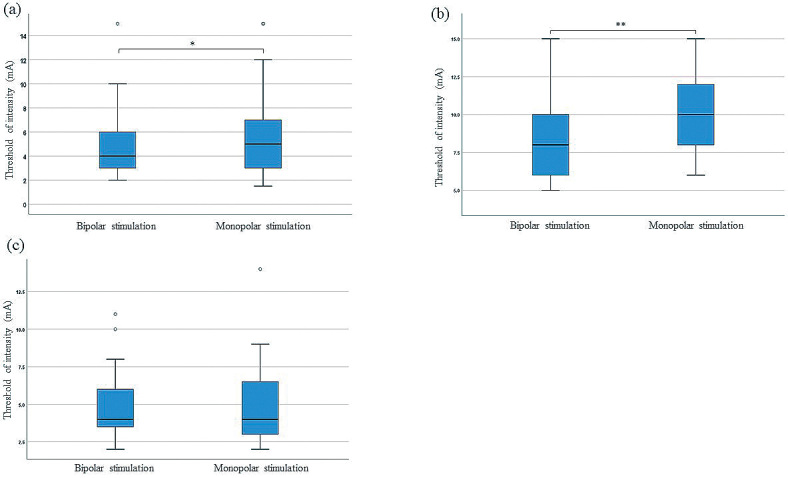
Box plots of the threshold for each clinical sign for bipolar and monopolar cortical stimulation methods.
(a) The threshold for bipolar stimulation to induce a motor response was significantly lower than that for monopolar stimulation. * p < 0.05.
(b) The threshold for bipolar stimulation to induce a language response was significantly lower than that for monopolar stimulation. ** p < 0.0005.
(c) There was no significant difference between the thresholds required for bipolar and monopolar stimulation to induce a sensory response.
Language symptoms were elicited by 50 electrodes in 10 patients. The thresholds of bipolar stimulation were lower than monopolar stimulation in 29 electrodes (58%), comparable in 14 electrodes (28%), and higher in 7 electrodes (14%). The median stimulation intensity threshold to produce language symptoms was 8 mA (IQR: 6-10 mA) for bipolar stimulation and 10 mA (IQR: 8-12.25 mA) for monopolar stimulation. Statistical analyses revealed that the threshold for monopolar stimulation was significantly higher than that for bipolar stimulation (N = 50, p < 0.0005) (Fig. 4b).
Sensory symptoms were induced by 19 electrodes in 8 patients. The thresholds of bipolar stimulation were lower than monopolar stimulation in 4 electrodes (21%), comparable in 8 electrodes (42%), and higher in 7 electrodes (37%). The median stimulation intensity threshold to produce sensory symptoms was 4 mA (IQR: 3-6 mA) for bipolar stimulation and 4 mA (IQR: 3-7 mA) for monopolar stimulation. There was no significant difference between the two stimulation methods (N = 19, p = 0.474) (Fig. 4c).
Discussion
Our study revealed that bipolar stimulation required a lower intensity to induce motor and language symptoms than monopolar stimulation, whereas there was no significant difference between the two stimulation methods in the required intensity to elicit sensory symptoms.
A previous report compared two modes of stimulation (bipolar vs monopolar) used to induce clinical motor responses in five patients with intractable frontal lobe epilepsy.5) The authors reported that bipolar cortical stimulation required less stimulation current to elicit clinical signs with a higher propensity to produce afterdischarges. The present results are concordant with this previous study. Furthermore, our results also showed the same tendency in the language symptoms. Nathan et al. revealed, with the use of a 3-dimensional finite model of the human brain, that bipolar direct cortical stimulation was more effective in producing localized current flows between adjacent electrodes than monopolar stimulation.4) In contrast, the current density of monopolar stimulation was distributed concentrically and widely around the electrode grid, but rapidly decreased with depth compared with bipolar stimulation. Based on these simulated data, we speculate that bipolar stimulation produces higher current densities on the local surface of the cerebral cortex; thus, even low current intensities could induce motor and language symptoms. The higher propensity of afterdischarges in bipolar stimulation of the previous report can also be explained by the denser current flow.5)
Our study resulted that there was no significant difference between the abilities of bipolar and monopolar stimulation to induce sensory responses. Several possible reasons could be considered for this result. First, this study showed that the threshold to induce a sensory response was lower than for other clinical responses, which is concordant with previous reports.6,7) Therefore, the threshold difference of two modes of stimulation might be subtle and difficult to detect using the 1-mA-step increments of our protocol. Second, the number of electrodes placed over the sensory cortex was limited in our patient group, and the number of electrodes used to induce sensory signs might not be sufficient to detect a statistical significance. Accumulation of more cases evaluated using finer stimulation step increments is required for further confirmation.
Our study also clarified the characteristics of the two stimulation methods. Bipolar stimulation provides a high local charge density and can easily elicit clinical symptoms but may have inferior spatial resolution, due to the simultaneous stimulation of adjacent two electrodes. In addition, bipolar stimulation has the limitation of evaluating the outer row and edge of the electrode array.5) In contrast, monopolar stimulation has higher spatial resolution than bipolar stimulation but may require higher stimulus thresholds than bipolar stimulation. As mentioned above, in our protocol, all electrodes were screened using bipolar stimulation, and then the eloquent pairs were stimulated using monopolar stimulation for more precise localization. Taking into account the lower threshold of bipolar stimulation, we suggest that bipolar stimulation would be more sensitive and suitable for screening and higher spatial resolution of monopolar stimulation would be suitable for precise localization. Bipolar stimulation alone may be difficult to determine finer localization, while monopolar stimulation alone may be time-consuming and may have a high stimulation threshold; therefore, our method of combining two ways would be effective for reliable and precise functional mapping.
Several limitations of this study need to be addressed. First, the research design is retrospective and the number of patients enrolled in this study was small. Inconsistencies in the tasks used, differences in the electrode locations and numbers, and additional pathologies may also have caused individual variation and biased the results. Accumulation of more cases is needed to confirm the present results and establish the validity of this stimulation protocol. Second, the effect of antiepileptic medication could not be eliminated and might affect the results. Antiepileptic medication at a usual maintenance dose was restarted during electrical cortical stimulation in our protocol. These antiepileptic drugs are effective in preventing seizures caused by cortical stimulation,8,9) but also might increase the threshold to elicit a clinical response. Third, the locations of the eloquent cortices were near the epileptogenic lesions, and the effect of epileptogenicity could not be eliminated.
Despite these limitations, this study revealed that bipolar cortical stimulation required a lower intensity to produce clinical signs of motor and language symptoms and thus would be suitable method for screening of the eloquent area in functional mapping. On the other hand, monopolar stimulation has higher spatial resolution and would be suitable method for more precise localization.
Conflicts of Interest Disclosure
The authors declare no conflicts of interest associated with this manuscript.
References
- 1). Hamberger MJ, Williams AC, Schevon CA: Extraoperative neurostimulation mapping: results from an international survey of epilepsy surgery programs. Epilepsia 55: 933-939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Motamedi GK, Okunola O, Kalhorn CG, et al. : Afterdischarges during cortical stimulation at different frequencies and intensities. Epilepsy Res 77: 65-69, 2007 [DOI] [PubMed] [Google Scholar]
- 3). Lesser RP, Luders H, Klem G, Dinner DS, Morris HH, Hahn J: Cortical afterdischarge and functional response thresholds: results of extraoperative testing. Epilepsia 25: 615-621, 1984 [DOI] [PubMed] [Google Scholar]
- 4). Nathan SS, Sinha SR, Gordon B, Lesser RP, Thakor NV: Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalogr Clin Neurophysiol 86: 183-192, 1993 [DOI] [PubMed] [Google Scholar]
- 5). Kovac S, Scott CA, Maglajlija V, et al. : Comparison of bipolar versus monopolar extraoperative electrical cortical stimulation mapping in patients with focal epilepsy. Clin Neurophysiol 125: 667-674, 2014 [DOI] [PubMed] [Google Scholar]
- 6). Lüders HO, Tanner AS: Cortical mapping by electrical stimulation of subdural electrodes: primary somatosensory and motor area, Lüders H (ed): Textbook of epilepsy surgery. London, Taylor & Francis, 2008, pp 978-982 [Google Scholar]
- 7). Kanno A, Enatsu R, Ookawa S, Ochi S, Mikuni N: Location and threshold of electrical cortical stimulation for functional brain mapping. World Neurosurg 119: e125-e130, 2018 [DOI] [PubMed] [Google Scholar]
- 8). Hoogerkamp A, Vis PW, Danhof M, Voskuyl RA: Characterization of the pharmacodynamics of several antiepileptic drugs in a direct cortical stimulation model of anticonvulsant effect in the rat. J Pharmacol Exp Ther 269: 521-528, 1994 [PubMed] [Google Scholar]
- 9). Hampel KG, Gomez-Ibanez A, Garces-Sanchez M, et al. : Antiepileptic drug reduction and increased risk of stimulation-evoked focal to bilateral tonic-clonic seizure during cortical stimulation in patients with focal epilepsy. Epilepsy Behav 80: 104-108, 2018 [DOI] [PubMed] [Google Scholar]