Abstract
Aims
To evaluate the impact of a simplified, rapid cardiovascular magnetic resonance (CMR) protocol embedded in care and supported by a partner education programme on the management of cardiomyopathy (CMP) in low- and middle-income countries (LMICs).
Methods and results
Rapid CMR focused particularly on CMP was implemented in 11 centres, 7 cities, 5 countries, and 3 continents linked to training courses for local professionals. Patients were followed up for 24 months to assess impact. The rate of subsequent adoption was tracked. Five CMR conferences were delivered (920 attendees—potential referrers, radiographers, reporting cardiologists, or radiologists) and five new centres starting CMR. Six hundred and one patients were scanned. Cardiovascular magnetic resonance indications were 24% non-contrast T2* scans [myocardial iron overload (MIO)] and 72% suspected/known cardiomyopathies (including ischaemic and viability). Ninety-eighty per cent of studies were of diagnostic quality. The average scan time was 22 ± 6 min (contrast) and 12 ± 4 min (non-contrast), a potential cost/throughput reduction of between 30 and 60%. Cardiovascular magnetic resonance findings impacted management in 62%, including a new diagnosis in 22% and MIO detected in 30% of non-contrast scans. Nine centres continued using rapid CMR 2 years later (typically 1–2 days per week, 30 min slots).
Conclusions
Rapid CMR of diagnostic quality can be delivered using available technology in LMICs. When embedded in care and a training programme, costs are lower, care is improved, and services can be sustained over time.
Keywords: Rapid cardiac MRI, Education, Abbreviated protocols, Low–middle-income countries, Cardiomyopathy, Impact on management
Structured Graphical Abstract
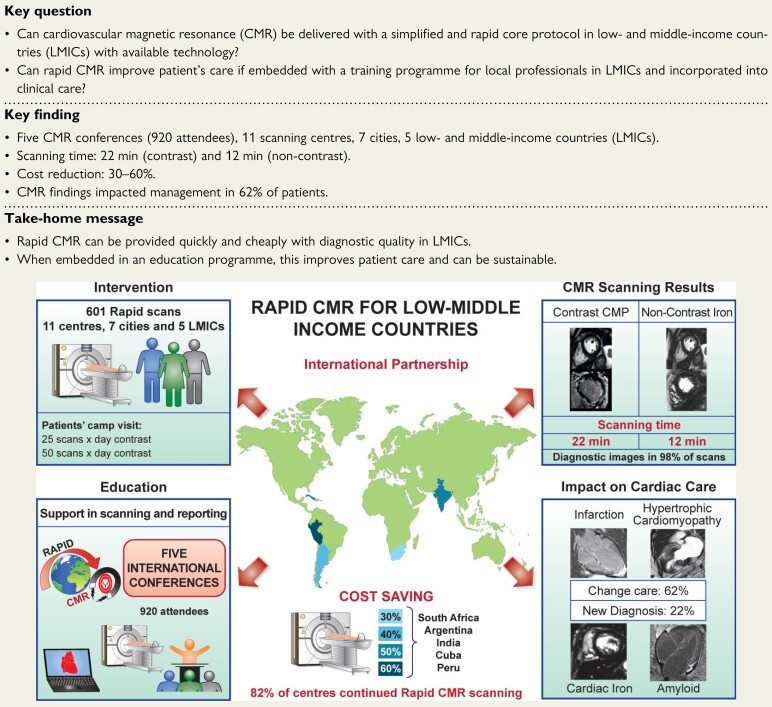
Structured Graphical Abstract.
Rapid cardiovascular magnetic resonance (CMR) is an international partnership project that aimed to evaluate the impact of a simplified and less expensive CMR protocol embedded with an educational programme to assess a wide spectrum of cardiomyopathies in 11 centres from national/state capital in five low- and middle-income countries (LMICs) with available technology. Over a 5-year period, we delivered an education programme to 920 referrers. Six hundred and one patients were scanned, reducing the time of scanning to <22 min, potentially saving costs and impacting on patients’ care. Our project highlights the potential and beneficial role of rapid CMR in LMICs. CMR, cardiovascular magnetic resonance; LMICs, low- and middle-income countries; CMP, cardiomyopathy.
See the editorial comment for this article ‘Advancing population-scale access to high-value cardiovascular care: a roadmap for CMR and beyond’, by Subha V. Raman, https://doi.org/10.1093/eurheartj/ehac079.
Introduction
Optimizing cardiovascular care relies on the availability of diagnostic testing. Test appropriateness depends on factors, including disease prevalence, pre-test probability, therapeutic options, test performance, availability, and cost.1 Limited testing may be a barrier to optimal care in low- and middle-income countries (LMICs), where cardiovascular disease is a leading cause of mortality and morbidity.2 Echocardiography is the first-line diagnostic test for cardiac patients,3 but in isolation may not be sufficient. Cardiovascular magnetic resonance (CMR) complements echocardiography4–6 and is recommended in multiple international guidelines, including the majority from the European Society of Cardiology (ESC) (14 guidelines, 39 Class I, and 22 Class II recommendations).7 It is the gold standard for the evaluation of ventricular function,8 scar imaging using late gadolinium enhancement (LGE)9 and iron quantification (CMR-T2*).10 Where CMR-T2* has been adopted to guide chelation therapy for transfusion-dependent thalassaemic patients, a >70% reduction in cardiovascular mortality has been reported.11 Cardiovascular magnetic resonance also plays a critical role in quantifying changes in myocardial composition based on T1, T2 relaxation times, and extracellular volume,12 and it detects ischaemic heart disease, having shown to be superior to other imaging techniques.13
Cardiovascular magnetic resonance has been economically limited to high-income countries (HICs).14,15 Access is very limited in LMICs despite magnetic resonance imaging (MRI) availability,16 though CMR would likely have utility in the major LMIC tertiary healthcare centres, for example, where device therapy, angioplasty, or cardiac surgery are available but need optimal targeting to optimize resource utilization. There are several barriers to adoption, including negative perception (that CMR scans are expensive, time-intensive, and complex), a lack of local expertise (poor access to training for service deliverers and referrers), insufficient investment (overall health sector, private/public sector distribution, poor inter-sector co-ordination),17 a lack of radiology and cardiology co-ordination, and competition for MRI access.
We aimed to make CMR available in LMICs and to assess its utility. Over 5 years in two pilot studies (TIC-TOC18 and INCA-Peru19), we developed and demonstrated rapid CMR in LMICs, initially for cardiac iron quantification in thalassaemic patients (Bangkok, Thailand) with an 8 min protocol18 and then contrast CMR for cardiomyopathy (CMP) evaluation (two centres, Lima, Peru) with an 18 min protocol.19 These demonstrated the technical feasibility of rapid CMR and highlighted the importance of system-wide change, embedding imaging in cardiac care with an educational programme and a supportive mentoring environment for emerging services. To explore this further, we developed the rapid CMR in LMICs project (www.rapidcmr.com) and performed abbreviated CMR scans in 11 centres, 7 cities, 5 countries, and 3 continents. We used large centres in national capital cities but also involved smaller centres in state capitals. We aimed to embed rapid CMR within clinical care pathways in order to improve sustainability and utilization by supplementing scanning with an educational programme and mentoring local staff who tracked patient outcomes.
Methods
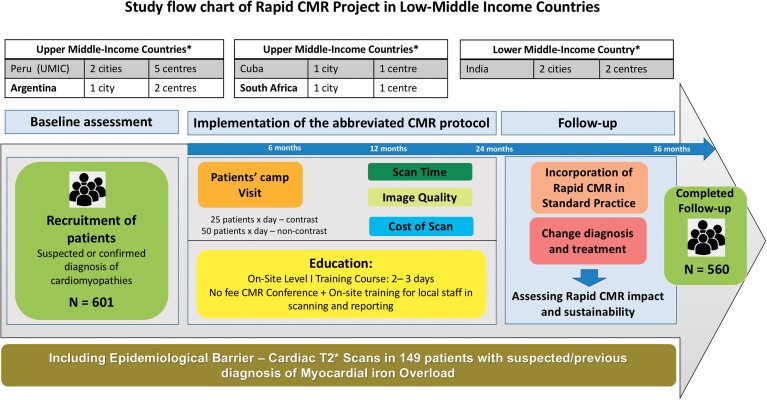
This is a multicentre, prospective observational cohort study (Figure 1). The research complied with the Declaration of Helsinki and received ethical approval in the UK—University College London (REC 11255/001 and 11255/002) and by the relevant local ethics committees in participating centres. An international partnership was established between UK-UCL and Barts Heart Centre, London, UK, and each participating centre, with the support of the local cardiology and radiology societies (that supported advertising the rapid CMR in their channels to local professionals), local scientific councils, and British embassies in Argentina, Peru, and Cuba (who co-financed the UK team visit), and the Society for Cardiovascular Magnetic Resonance (SCMR) (who sponsored one to two SCMR educators/trainers and provided no-fee SCMR certification for participants). A team of five doctors, experts in the field (UK, SCMR expert delegates) travelled for each visit to the participating countries to deliver an educational programme to local doctors and train/partner with local personnel to help perform assessments and support rapid CMR scanning. When possible, we additionally included experts from nearby countries with native language capabilities and encouraged the development of local networks for sustainability and mentorship.
Figure 1.
Rapid cardiovascular magnetic resonance project, design of the project, and implementation. Eleven hospitals with magnetic resonance imaging scanners and cardiac imaging department in seven cities and five countries, with 601 patients enrolled in the study and followed up for a median time of 24 months to assess the impact and sustainability of the abbreviated cardiovascular magnetic resonance protocol. *World Bank classification of upper- and low–middle-income countries, based on the gross national income per capita.
Study implementation
Patients were recruited by the local research teams. All participants provided written consent. The rapid CMR contrast demonstrator study of 98 patients scanned in two centres in Peru were previously published19 who remain included but with their 36-month follow-up and in multiple cases (iron overload, cardiotoxicity) interval scanning. Following this pilot project, 10 additional participating centres contacted our research group as they wanted to initiate/improve their efficiency in CMR scans. There was one dedicated cardiac CMR scanner (Cuba, 3 days per week), five scanners with one-day-a-week CMR (Peru—one centre, South Africa—one Centre, Argentina—two centres, India—one centre), and five scanners performing no CMR before initiation of the project (Peru—four centres and India—one centre). Scanner time was secured mainly during unused magnet time (weekends, national holidays) and the international delegation visit, educational programme, and ‘patients’ camp’ visits were scheduled accordingly. Scans were at no cost to participants and the costs were borne by the participating centre (scanner availability, staff support) and the research funding (providing contrast agent and ECG). The patients’ inclusion criteria are cited in Supplementary material online, Appendix—Table S1.
Education and training programme
Education was a key component of our project, and it was delivered at two levels:
CMR International Conferences: These were 2–3 days on CMR scanning and reporting per country for local cardiologists, radiologists, and radiographers. All were free of charge. Society for Cardiovascular Magnetic Resonance provided free Level 1 SCMR certification20 for participants and a free 1-year SCMR membership. CMR teaching included lectures by local/national/international speakers, direct CMR scanning observation, and a Level 1 reporting course.
Training for those delivering CMR: Six CMR centres were already established pre the rapid CMR intervention. Five were novice. For these, one to two international visiting doctors/radiographers delivered training in scanning and reporting to the local team days before the main intervention. For both, there was support during scanning days. Follow-up continuous support was given to local doctors remotely (technical/interpretation of difficult cases). We assessed the impact by measuring sustainability—the number of scans (and duration) delivered after the intervention period.
Abbreviated cardiovascular magnetic resonance protocol and implementation
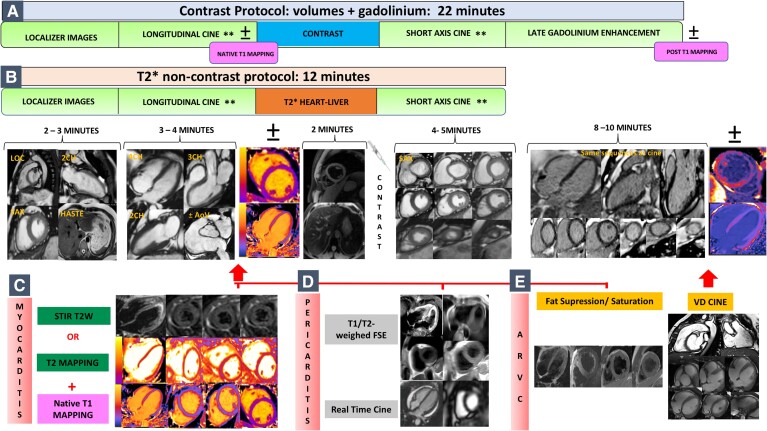
The rapid CMR protocol was published previously (INCA-Peru study).19 Here, we modified for specific patients (e.g. patient unable to perform breath holds adequately, patients with arrhythmia), and additional sequences were added for specific cardiac pathologies following SCMR sequence recommendations dependent on the scan indication8 (Figure 2). We followed a standardized workflow to maximize efficiency surrounding CMR (see Supplementary material online, Appendix—Table S2). One to two weeks before the main intervention, the rapid CMR protocol was shared remotely and installed in the local MRI scanner (with the support of the local radiographers/MRI engineering service). Preliminary scanning was done in healthy volunteers in each participating centre and mentored via live webcasting.
Figure 2.
Abbreviated cardiovascular magnetic resonance protocol: (A) 22 min contrast cardiovascular magnetic resonance protocol for the assessment of cardiomyopathies, with the option to add T1 mapping sequence if available in the scanner; (B) 12 min T2* non-contrast protocol to assess myocardial iron overload. Extra sequences added for specific pathologies: (C) short tau inversion recovery T2w or T2 mapping + T1 mapping for myocarditis (27 min scan); (D) T1 or T2w FSE + real-time cine for pericarditis (27 min scans); and (E) fat suppression and saturation + right ventricle cine acquisition for arrhythmogenic right ventricular cardiomyopathy (30 min scan). **If poor breath-holders, modification of the protocol using parallel imaging for cine images acquisition and in patients with arrhythmia, prospective triggering, and real-time cine imaging sequences acquired. CMR, cardiovascular magnetic resonance; MIO, myocardial iron overload; STIR, short tau inversion recovery; FSE, fast/turbo spin echo; RV, right ventricle; ARVC, arrhythmogenic right ventricular cardiomyopathy.
Standard cardiomyopathy cardiovascular magnetic resonance protocol
Localizers: A pilot three-plane localizer, a transverse bright blood stack for anatomic evaluation (optional ungated); pilots two-chamber and short-axis (SAX) stack.
Longitudinal axis: Steady-state free precession (SSFP) cine (25 phases) in four-, two-, and three-chamber views.
Hand contrast injection (dose 0.1 mmol/kg) gadoterate meglumine/gadobutrol.21
SAX cine stack and aortic valve cine.
LGE: repeating cine views as needed, followed by an inversion time scout if needed, then a segmented k-space LGE acquisition in multiple planes with a phase-sensitive inversion recovery sequence and magnitude reconstructions.
Additional sequences (if available in the scanners): for specific clinical indications including:8
For all cardiomyopathies: a single T1 mapping mid-SAX slice and four-chamber pre- and post-contrast (10 min after contrast administration).
-
For arrhythmogenic right ventricular CMP (ARVC):
Transaxial or oblique transaxial bSSFP cine images (slice thickness 5–6 mm) covering the right ventricle, including the right ventricular outflow tract.
Selected black blood images (four-chamber and three SAX slices: base, mid, and apex) + repeat same geometry with fat suppression.
For myocarditis: Selected T1 mapping, T2 mapping, or T2w STIR sequences at four-chamber and three SAX slices: base, mid, and apex.
-
For pericardial disease:
T1 or T2-weighted fast/turbo spin echo (FSE) images in one representative long-axis and one representative SAX slice to measure pericardial thickness.
Real-time cine imaging during dynamic breathing manoeuvres for evaluation of ventricular interdependence (mid-ventricular SAX).
For myocardial and liver iron overload (non-contrast scan): a T2* multi-echo gradient-echo transversal single slice trying to cover the maximum liver parenchyma and one single mid-cardiac SAX slice.
In patients with arrhythmia and poor breath-holder patients, we modified the protocol and used parallel imaging, prospective triggering, and real-time cine imaging. All these sequences were available in the participating MRI centres.
Image reporting
Images were analysed using cvi42 (Circle Cardiovascular Imaging Inc., Version 5.11.4–1559, Calgary, Canada) with a provided typically 3-month ongoing licence to participating centres (two centres had pre-existing CVI42 licences). Scans were reported the same day by the local doctor, supported, and reviewed by the international visiting experts (final report signed-off by a doctor with a Level 2 or 3 CMR EACVI—ESC certification or equivalent).20 After the onsite intervention, the participating centres used other software (e.g. Osirix, ARGUS, and free online post-procession software for cardiac and iron T2* analysis—http://www.isodense.com/mcdcm/mviewer.html). Reporting was used as a training exercise during the educational conference, where indications for the scan and the anonymized images were shown and discussed with the attendees after the patients’ consent. Reports were translated into the local language where needed and incorporated into local medical records.
Image quality
Image quality was assessed by a CMR expert (Level 3 EACVI-ESC certified).20 The criteria used were not the presence or absence of artefacts but whether images acquired could answer the clinical question posed: poor quality (unable to answer the clinical question), moderate quality (artefact but still interpretable), and good quality (optimal quality, definitive assessment).
Study outcome measures
Patients were followed up for a median time of 24 months by their local physician. Cardiovascular magnetic resonance-induced management changes were reported if CMR resulted in: (i) new diagnosis (of a pathology not suspected before), (ii) medication change, (iii) interventional treatment, and (iv) hospital admission or discharge.
Cost evaluation
Local CMR reference costs were assessed individually, as healthcare infrastructure, systems, and costs differed among countries. In general, we considered as reference the average cost from public and private hospitals offering CMR at the time of scanning. Costs included: hospital CMR charges, contrast, intravenous cannula/tubing, ECG electrodes, physician (reporting), nurse, and radiographer fees.
Statistical analysis
Data were analysed using SPSS (version 24·0, Statistical Package for the Social Sciences, International Business Machines, Inc., Armonk, NY, USA). Continuous data were expressed as mean with standard deviation, and categorical data are presented as absolute numbers and percentages. Normal distribution was formally tested using the Shapiro–Wilk test. Continuous data were compared using two-sided Student’s t-tests, and categorical variables were compared using χ 2-test or Fisher’s exact test as appropriate. Statistical significance was defined as a two-sided P-value of <0.05.
Results
Baseline characteristics
Overall, 601 patients were referred from 14 hospitals, seven cities in five countries as follows: Peru, Lima, and Arequipa, seven centres, n = 261; Argentina, Buenos Aires, three centres, n = 69; Cuba, La Havana, one centre, n = 114; South Africa, Cape Town, one centre, n = 34 and India, Delhi, and Jaipur, two centres, n = 123. A total of 449 (74%) were referred by cardiologists, 131 (22%) by haematologists [myocardial iron overload (MIO) indication], and 20 (3%) by other specialists. The mean age of the patients was 46 ± 19 years with suspected iron overload patients being younger (28 ± 13 vs. 53 ± 16 years, P < 0.001, 95% of MIO scans were of patients with beta-thalassaemia); 56% of participants were male (Table 1).
Table 1.
Baseline characteristics of the patients enrolled in the rapid cardiovascular magnetic resonance project
| Clinical data | Total | Non-contrast CMR scans | Contrast CMR scans | P-value |
|---|---|---|---|---|
| Demographic | ||||
| Total | 601 (100%) | 154 (26%) | 446 (74%) | – |
| Age, years (mean ± SD) | 46 ± 19 | 28 ± 13 | 53 ± 16 | <0.001 |
| Female sex | 267 (44%) | 82 (53%) | 185 (41%) | 0.01 |
| BMI, kg/m2 (mean ± SD) | 25.2 ± 5.4 | 21.4 ± 3.7 | 26.1 ± 5.2 | 0.02 |
| BSA, m2 (mean ± SD) | 1.7 ± 0.3 | 1.5 ± 0.2 | 1.8 ± 0.2 | 0.9 |
| Imaging modalities used before CMR | ||||
| Echocardiography | 471 (78%) | 51 (33%) | 420 (94%) | <0.001 |
| CTCA | 46 (8%) | 0 | 46 (10%) | <0.001 |
| SPECT/bone scan | 55 (9%) | 0 | 55 (12%) | <0.001 |
| CMR | 85 (14%) | 48 (31%) | 37 (8%) | <0.001 |
| Referral | ||||
| Cardiologist | 449 | 21 | 428 | <0.001 |
| Haematologist | 131 | 131 | 0 | <0.001 |
| Other | 20 | 0 | 20 | <0.001 |
CMR, cardiovascular magnetic resonance; BMI, body mass index; BSA, body surface area; SD, standard deviation; CTCA, computed tomography coronary angiography; SPECT, single photon emission computed tomography.
Despite the intention to concentrate on the assessment of cardiomyopathies, referrals included other cardiac conditions deemed urgent, usually involving economically disadvantaged patients who would normally not have access to this resource.
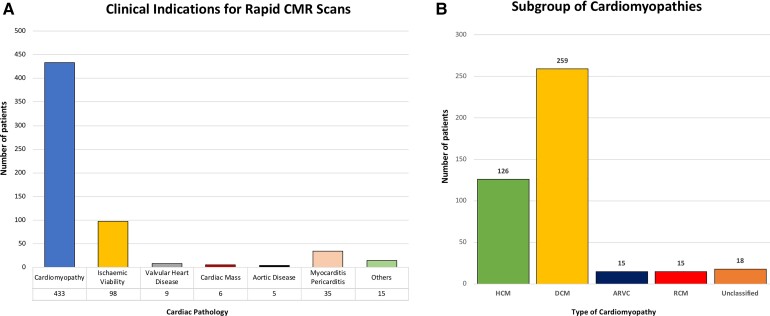
Reasons for CMR referrals were: cardiomyopathies in 433 (72%), assessment of viability in 98 (16%), myocarditis/pericarditis in 35 (6%), valvular heart disease in 9 (1%), cardiac tumours in 6 (1%), aortic disease in 5 (1%), and other indications (e.g. congenital) in 15 (2%). Within the group of cardiomyopathies, the indications were: dilated CMP in 259 (60%), hypertrophic CMP (HCM) in 126 (29%), ARVC in 15 (3%), restrictive CMP in 15 (3%), and unclassified in 18 (4%). Eighteen different pathologies were assessed (Figure 3).
Figure 3.
Clinical indications for the use of the abbreviated cardiovascular magnetic resonance protocol in (A) all cardiac pathologies and (B) within the group of cardiomyopathy sub-types. ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy.
Cardiovascular magnetic resonance results
Patients were scanned in 11 hospitals with MRI units, using 14 different scanners (six models from three manufacturers at two field strengths, with scanner age at study time of 12 ± 3.4 years) (see Supplementary material online, Appendix—Table S3).
One hundred and fifty-four (26%) patients had a non-contrast scan, 149 (96%) for MIO, four other patients had chronic kidney disease, and hence contrast was not administered, and one patient declined contrast. For contrast CMR scanning, 410 (94%) had undergone transthoracic echocardiography prior to the CMR scan, 10% computed tomography coronary angiography, 12% single photon emission computed tomography, and 8% had a previous CMR. Within the group of patients referred for a non-contrast T2* CMR scan to assess MIO, 31% had previously undergone CMR to assess cardiac iron. Among the 601 scans, 98% were of diagnostic quality.
Good image quality was reported in 506 scans (84%), moderate in 85 (14%), and poor in 10 (2%). Poor image quality was due to atrial fibrillation with rapid ventricular response (n = 5), very poor breath-holding (n = 3), and frequent ventricular ectopic beats (n = 2). Moderate image quality was noted with arrhythmia (n = 58) and poor breath holds (n = 27).
The delivered throughput was 50 scans per scanner day for iron T2* CMR with typically ∼10 h day and 25 scans per scanner day for contrast CMP. Scan time (average ± standard deviation) was 12 ± 4 min for T2* for MIO and 22 ± 6 min for the basic CMP CMR protocol (function + contrast). In 32 (5%) of the CMP scans, pre- and post-T1 mapping sequences were included with an average scan time of 25 ± 5 min. For other specific clinical indications, CMR scanning time took longer as additional sequences were added: myocarditis/pericarditis protocol (STIR and T2 FSE or T1/T2 mapping if available) 27 ± 4 min and ARVC protocol (with RV cine + fat suppression sequences) 30 ± 10 min. Within the T2* CMR scans, 45 (30%) of patients had cardiac iron (cardiac T2* <20 ms); 36 (80%) previously undocumented. For contrast CMR scans, 288 (64%) patients had positive LGE (for some diseases, such as HCM, LGE was often extensive on qualitative assessment). Thirty-six (8%) were considered normal or non-conclusive studies (Table 2).
Table 2.
Time of scanning for the rapid cardiovascular magnetic resonance protocol
| Type of rapid CMR protocol for the detection of cardiomyopathies | |||||
|---|---|---|---|---|---|
| Iron CMR
(cine + T2*) n = 149 |
CMP
(cine + LGE) n = 350 |
CMP mapping (cine + LGE + T1 mapping) n = 32 |
Myocarditis/pericarditis (STIR/T1 FSE or
mapping if available) n = 35 |
ARVC (RV cine
fat Sat/Sup.) n = 15 |
|
| Age, years (mean ± SD) | 26 ± 11 | 54 ± 16 | 47 ± 16 | 48 ± 19 | 30 ± 10 |
| Time, min (mean ± SD) | 12 ± 4 | 22 ± 6 | 25 ± 5 | 27 ± 4 | 30 ± 10 |
| Image quality | |||||
| Good | 129 (87%) | 294 (84%) | 28 (87%) | 29 (82%) | 12 (82%) |
| Moderate | 17 (11%) | 49 (14%) | 4 (13%) | 6 (18%) | 3 (18%) |
| Poor | 3 (2%) | 7 (2%) | 0 (0%) | 0 | 0 |
| Arrhythmia | 2 (1%) | 48 (14%) | 4 (13%) | 0 (0%) | 4 (27%) |
Contrast CMR protocol for cardiomyopathies and non-contrast CMR scan to assess myocardial iron overload. Modification of the protocol and time of scanning for specific cardiac pathologies. CMR, cardiovascular magnetic resonance; ARVC, arrhythmogenic right ventricular cardiomyopathy; CMP, cardiomyopathy; LGE, late gadolinium enhancement; STIR, short tau inversion recovery; FSE, fast/turbo spin echo; RV, right ventricle; SD, standard deviation; Sat/Sup, saturation and suppression.
Cardiovascular magnetic resonance impact on diagnosis and management
A total of 560 (93%) patients completed follow-up (median 24 months post-CMR). Forty-one participants were not contactable. The abbreviated CMR protocol changed clinical management in 62%, either by revealing a new diagnosis (22%) or by leading to a change in therapy (40%) (Figure 4, for new diagnosis—contrast rapid CMR scans and Supplementary material online, Appendix S4 for three clinical case examples). The T2* CMR scans (cardiac and liver assessment) largely led to a change/start of chelation therapy in 55% of patients, as the diagnosis of transfusion-dependent thalassaemia was already established. After rapid CMR, 12% with cardiomyopathies required further invasive tests to arrive at their final diagnosis (e.g. angiography, cardiac biopsy) or to therapeutic interventions (e.g. implantable-cardioverter defibrillator) (Table 3). Although non-blinded, rapid CMR did not miss any findings previously made by echocardiography.
Figure 4.
Clinical indications for the use of the abbreviated contrast rapid cardiovascular magnetic resonance protocol to assess ischaemic/non-ischaemic cardiomyopathies and myocarditis/pericarditis. After a 24-month follow-up period, 89 new diagnoses were made. CMR, cardiovascular magnetic resonance; CMP, cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVH, left ventricular hypertrophy; MINOCA, myocardial infarction with non-obstructive coronary arteries; ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy.
Table 3.
Impact of the rapid cardiovascular magnetic resonance protocol on patient care
| CMR findings | Cohort | Cardiac T2* iron scans | Contrast CMR scans | P-value |
|---|---|---|---|---|
| Abnormal baseline CMR results | ||||
| No. | 601 (100%) | 149 (25%) | 452 (75%) | – |
| Cardiac iron overload (T2* <20 ms) | 45 (7%) | 45 (30%) | N/A | – |
| Presence of LGE | 288 (48%) | N/A | 288 (64%) | – |
| Inconclusive CMR | 10 (2%) | 0 (0%) | 10 (2%) | <0.001 |
| Follow-up—impact on patient care | ||||
| No. | 560 (100%) | 129 (23%) | 431 (77%) | |
| New diagnosis | 125 (22%) | 36 (28%) | 89 (21%) | 0.19 |
| Change/addition of new medication | 134 (24%) | 36 (28%) | 98 (23%) | <0.001 |
| Intervention or surgery | 46 (8%) | 0 (0%) | 46 (8%) | <0.001 |
| Coronary angiography or biopsy | 21 (4%) | 0 (0%) | 21 (4%) | <0.001 |
| Hospital discharge and admission | 25 (4%) | 0 (0%) | 25 (6%) | <0.001 |
| Other imaging modalities requested after CMR to support diagnosis and therapy | ||||
| No. | 560 (100%) | 129 (23%) | 431 (77%) | – |
| Echocardiography | 45 (8%) | 3 (2%) | 42 (9%) | <0.001 |
| CTCA | 17 (3%) | 0 (%) | 17 (3%) | <0.001 |
| SPECT | 12 (2%) | 0 (%) | 12 (2%) | <0.001 |
CMR findings during patients’ camp scanning and assessment of the impact of the abbreviated CMR protocol on patients’ care and further additional cardiac imaging requested to support patients’ care. CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; CTCA, computed tomography coronary angiography; SPECT, single photon emission computed tomography.
Cost evaluation
Estimated cost savings for rapid compared with conventional contrast CMR were between 30 and 60% (equivalent to one-third to two-thirds cheaper) with a range of 25–30% in Cape Town, 30% in Argentina, 40% in Cuba, and 50–60% in Peru. For non-contrast T2* CMR, the saving ranged between 40% in India and 50% in Peru (Table 4).
Table 4.
Cardiovascular magnetic resonance practice in the participating centres before and after the implementation of the rapid cardiovascular magnetic resonance protocol
| Country | Argentina | Cuba | Peru | South Africa | India (non-contrast T2* CMR) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| City | Buenos aires (n = 69) | La havana (n = 114) | Lima (n = 248) | Arequipa (n = 13) | Cape town (n = 34) | Delhi (n = 53) | Jaipur (n = 70) | ||||
| Centre | Centre 1 | Centre 2 | Centre 3 | Centre 4 | Centre 5a | Centre 6 | Centre 7a | Centre 8 | Centre 9 | Centre 10 | Centre 11 |
| Before rapid CMR scan | |||||||||||
| Number of days per week | 1 | 2 | 3 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 |
| Number of hours × day | 5 | 5 | 5 | 0 | 5 | 0 | 0 | 0 | 5 | 2 | 0 |
| CMR scans per day | 4 | 5 | 3–4 | 0 | 4 | 0 | 0 | 0 | 5 | 2 | 0 |
| Cost | $400–$500 | $600 | $400–$600 | $350–$500 | $70–$100 | ||||||
| After rapid CMR scan | |||||||||||
| Number of days per week | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| Number of hours × day | 5 | 5 | 5 | 4 | 5 | 4 | 4 | 4 | 5 | 2 | 2 |
| CMR scans per day | 5–7 | 6–7 | 7–9 | 6 | 6–8 | 6 | 6 | 6 | 6–7 | 4–6 | 4–6b |
| Rapid CMR scans (3.0 min × contrast scan) (2.0 min × T2* scan | 5–6 | 3–4 | 6–8 | 4 | 4–6 | 4 | 4 | 4 | 5 | 4–6 | 4–6b |
| Cost rapid CMR scan | $280–$380 | $360 | $160–$300 | $200–$400 | $42–$60 | ||||||
| % saving | 30% | 40% | 50%–60% | 25–30% | 40% | ||||||
Argentina—Buenos Aires: Centre 1: Instituto IMAT. Centre 2: Hospital Italiano de Buenos Aires.
Cuba—La Havana: Centre 3: Instituto de Cardiologia y Cirugia Cardiovascular.
Peru—Lima: Centro 4: Hospital ESSALUD Edgardo Rebagliati. Centre 5: Hospital Central Militar. Centre 6: Hospital Central de la Fuerza Aerea del Peru. Centre 7: Hospital ESSALUD Guillermo Almenara.
Peru—Arequipa: Centre 8: Centro Salud Cerema.
South Africa—Cape Town: Centre 9: Groote Schuur Hospital in Cape Town.
India—Delhi: Centre 10: Mahajan Imaging Centre.
India—Jaipur: Centre 11: OK Diagnostic Imaging Centre.
Faulty scanners, stopped CMR service (the number representing post-study to pre-breakdown).
Ten scans done after rapid CM camp visit. Starting that number of CMR scans this year.
Cardiovascular magnetic resonance education programme
Five international conferences were delivered in Peru (November 2016 and January 2019), Argentina (June 2018), Cuba (November 2019), and South Africa (June 2018). Each conference had at least 180 attendees, with a total of 920 professionals. All completing participants received no-fee Level 1 SCMR certification and free SCMR membership. See www.rapidcmr.com for prior course details.
A total of 18 professionals were trained in CMR scanning and reporting (1–2 professionals per centre—7 doctors and 11 radiographers in total). Thirteen out of 18 professionals are still using their skills, with three doctors achieving a formal CMR training (equivalent to Level 2 CMR certification), but five professionals (three radiographers, one cardiologist, and one radiologist) did not continue CMR as the MRI units in two hospitals in Peru sustained permanent scanner breakdowns.
Subsequent adoption of rapid cardiovascular magnetic resonance
Nine out of 11 participating centres continued using rapid CMR (two hospitals in Peru ceased due to scanner breakdowns), with services scanning 1–2 days per week. From the entire list of CMR indications, the participating centres continue using the rapid CMR protocol to assess cardiomyopathies in 2–8 patients per day (Table 4). We followed up sites and found that the main services were maintained and even grew (Table 4) with a variety of different local funding solutions as the healthcare systems are complex and different for each country: (i) public (e.g. Peru, South Africa with CMR provided directly by public hospitals to government/non-government employees), (ii) Army/Navy (e.g. Peru), (iii) governmental (e.g. Cuba), (iv) private with third party payers (e.g. Argentina, Peru, with private hospital charging CMR scans to public hospitals), (iv) Charity Organization Societies (e.g. India, with T2* CMR scans costs co-financed by Thalassaemics India Patient Society—https://www.thalassemicsindia.org/).
Two new centres in two non-capital cities in Peru (Chiclayo and Huancayo) and one centre in La Havana, Cuba, initiated a CMR service (scanning 1/2 day per week) led by a CMR specialist physician with remote support from the international team of experts.
Discussion
We report the ability to perform rapid CMR in multiple LMICs in three continents across different clinical environments utilizing a variety of different scanners. These scans provided diagnostic image quality in the majority of cases at greatly reduced cost with an important impact on the care of the patient (Structured Graphical Abstract). This was achieved without having the most current scanner and software in many of the centres. To achieve this, four key strategies were employed: (i) a collaborative partnership between the investigators and local teams at multiple levels (a local healthcare champion, hospital team, political authorities, scientific societies, and embassies, (ii) CMR training and education at two levels: first, referring physician and second, to those providing CMR services (cardiologists, radiologists, and technologists—acquisition and reporting) in the local language or English with translation, (iii) a focused, rapid, and less expensive CMR scan with sequences adapted to local scanners and software, (iv) the integration of results into the care of the patient was with follow-up to assess the impact. The rate of subsequent adoption in the participating centres was tracked.
This is the first study that tackles the education barrier of CMR in LMICs, where most professionals need to travel overseas and self-fund for expensive courses to receive certified training.20 We provided training at the local participating sites ensuring that the appropriate technology is available. It is important to emphasize that while we provided training for practitioners (doctors and radiographers to deliver CMR), we made a large effort to train potential referrers by organizing the five Level 1 CMR international conferences, aiming that they can be enthusiastic about CMR, to learn more cardiology, and as a gateway for the (few) who want to take CMR further. Here, we had a ratio of basic advanced training of ∼50:1. The Level 1 training here is in no way wasted if they do not directly deliver CMR services but is perhaps the foundation of CMR incorporation into clinical practice.
Ninety-four per cent of patients had undergone previous echocardiography but needed CMR to complete their evaluation as there was equipoise regarding the diagnosis or appropriate therapy, which was not answered by previous imaging testing. Advanced tissue characterization with LGE allows for important risk stratification22 and estimation of prognosis.23 The extension of LGE seen in some cardiomyopathies such as HCM patients was far more than is typically seen in an HIC with an established CMR service. We believe this is a marker of a lack of access to advanced imaging. In LMICs, patients with more advanced stages of HCM were typically seen, suggesting that cases that might have been picked up by CMR during the early stages of the disease may have been missed. The referral indications for rapid CMR were mainly to assess cardiomyopathies and differed compared with HICs where heart failure, cardiomyopathies, function and viability, and stress perfusion are commonly indicated.24
The core structure of the abbreviated CMR protocol previously published in the INCA-Peru study19 to assess specific cardiomyopathies was modified for this study. Adding these sequences went out of the scope of our ‘keep it simple’ approach and this happened due to different challenges: individual patients outside our planned remit (congenital, masses, valvular), where to turn them down would give them no CMR option; also referrers and local champions some of who had trained overseas who demanded guideline appropriate scans,8 and in some cases of course ourselves. Even though we were delivering a research project, it proved difficult at times to not provide the same level of care we would in HIC parent institutions. It is interesting that the added sequences in many cases provided little incremental value (as discussed below for T1 mapping). Despite this, we showed that CMR can be acquired in 22 min on average for a core CMP protocol. Even when adding other sequences for specific pathologies, we were able to scan patients in <30 min. Overall, this corresponds to a reduction in scanning time of around 30–45% compared with reported CMR exam durations.8 The scanning time improved progressively once local radiographers gained experience (e.g. our second visit in Peru, the average scan time was 19 vs. 24 min for the first visit in Cuba).
Arrhythmias were detected in 10%, requiring protocol modification, but despite this, 98% of the scans were of diagnostic quality, similar to reports of conventional CMR scans from international surveys.14
In three centres (two in Peru and one in South Africa), T1 mapping was available, and we incorporated one mid-SAX and four-chamber view native and post-contrast T1 mapping—adding 3 min to the core protocol. T1 mapping provides incremental value in specific pathologies such as Fabry disease25 and cardiac amyloidosis26 without the need for contrast. Here, the (untargeted) yield was low, with none of these pathologies detected in 32 patients, although cardiac amyloidosis was found in some patients in the rapid core contrast CMR protocol.
The clinical utility of rapid CMR in affecting patient care was 62%, defined by a new or changed diagnosis, change in therapy, or subsequent procedure, similar to reports from international registries.14,15 Six per cent of our cohort was lost to follow-up. Many of those lost to follow-up lived in remote rural areas; we believe the impact of CMR in LMICs could be potentially higher than in HICs if these disadvantaged groups could be better tracked, although barriers to ongoing clinical management, including access to costly medications or devices, may limit this. Only 13% of our patients had additional cardiac imaging investigations triggered, mainly as part of the confirmation of a new diagnosis revealed by CMR, e.g. bone scintigraphy test to confirm cardiac amyloidosis.
Cost implications can only be broadly estimated due to different healthcare systems in the five countries. Our best estimate is that rapid CMR could reduce costs of contrast scans between one-third and two-thirds (equivalent to 30–60% of cost reduction). This does not include any cascading savings of better targeting of treatment. Further study would be needed to estimate this.
Further barriers remain: the lack of engineering and MRI maintenance service remains a barrier for service continuity (2 of the 11 centres ceased MRI because of this).
Although we focused on LMICs, there is potential utility in HICs. In the current COVID-19 pandemic,27 as an example, CMR needs to be done in a way that minimizes exposure to the staff without compromising the diagnostic yield of the study, as recently evaluate.28
Study limitations
This was not a randomized study. No assessment was made of prior services when present. The study was of the willing and able—included centres all had had a local, supported champion. The study was in either capital cities or state capitals within LMICs: results are not expected to generalize beyond this. Scanners were those that were available. Advanced CMR techniques (e.g. perfusion) were out of scope,13 even though recent data suggest these can also be done rapidly.29 Latest techniques were not used (e.g. compressed sensing).30 The team performed immediate CMR viewing, post-processing, and reporting in a way that would not necessarily be applicable to clinical services. No assessment on physician reimbursement, workloads, or whole care pathway costs was made. Our study does not fully address the barriers to a sustainable service, although ‘mission creep’ and a gradual increase in scanning times once the initial implementation is over was observed.
In conclusion, CMR core clinical information can be provided more quickly, easily, and less expensive. Our rapid CMR protocol can be delivered globally to major centres in LMICs (national/state capitals) and HICs alike. When incorporated into a clinical service and linked to an education/training programme with a supporting international network and partnership, it can deliver high diagnostic quality and improve patient care. The initial data suggest that these changes can be sustainable.
Supplementary Material
Acknowledgements
See Supplementary material online, S5 for the full list of acknowledgements. We acknowledge with grateful thanks for the support of the UK ambassadors to Peru and Cuba, Kate Harrison and Sir Antony Stokes, and their teams, respectively. We thank Thalassaemics India and its secretary Mrs Sobha Tuli for enabling the project in India to be undertaken. Finally, we are very grateful to all the patients, some of whom travelled long distances and took great effort to attend their CMR scans and for agreeing to participate in the study.
Contributor Information
Katia Devorha Menacho, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Sara Ramirez, Peruvian Air Force Hospital, Lima, Peru.
Aylen Perez, Cardiology and Cardiovascular Surgery National Institute, La Havana, Cuba.
Laura Dragonetti, High Technology Medical Institute IMAT, Buenos Aires, Argentina.
Diego Perez de Arenaza, Italian Hospital of Buenos Aires, Buenos Aires, Argentina.
Diana Katekaru, Military National Hospital, Cardiac Imaging Department, Lima, Peru.
Violeta Illatopa, National Cardiovascular Institute—INCOR, Lima, Peru.
Sara Munive, National Cardiovascular Institute—INCOR, Lima, Peru.
Bertha Rodriguez, Edgardo Rebagliati Hospital, MRI and CT Department, Lima, Peru.
Ana Shimabukuro, Guillermo Almenara Irigoyen Hospital, National Hospital, Lima, Peru.
Kelly Cupe, Guillermo Almenara Irigoyen Hospital, National Hospital, Lima, Peru.
Rajiv Bansal, Santokba Durlabhji Memorial Hospital Cum Medical Research Institute, Jaipur, India.
Vivek Bhargava, Okay Diagnostic Centre, Jaipur, India.
Ivonne Rodriguez, Sermedial Hospital, Arequipa, Peru.
Andreas Seraphim, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Kris Knott, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Amna Abdel-Gadir, Institute of Cardiovascular Science, University College London, London, UK.
Salomon Guerrero, Almanzor Aguinaga National Hospital, Chiclayo, Peru.
Marco Lazo, Ramiro Priale National Hospital, Huancayo, Peru.
David Uscamaita, Edgardo Rebagliati Hospital, MRI and CT Department, Lima, Peru.
Marco Rivero, Peruvian Air Force Hospital, Lima, Peru.
Neil Amaya, Edgardo Rebagliati Hospital, MRI and CT Department, Lima, Peru.
Sanjiv Sharma, AlI India Institute of Medical Sciences, New Delhi, India.
Amelia Peix, Cardiology and Cardiovascular Surgery National Institute, La Havana, Cuba.
Thomas Treibel, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Charlotte Manisty, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Sam Mohiddin, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Harold Litt, Department of Medicine (Cardiovascular Division), Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Yuchi Han, Department of Medicine (Cardiovascular Division), Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Juliano Fernandes, Jose Michel Kalaf Research Institute, Campinas, Brazil.
Ron Jacob, Lancaster General Health Hospital, Lancaster, USA.
Mark Westwood, St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Ntobeko Ntusi, Department of Medicine, University of Cape Town, Cape Town, South Africa.
Anna Herrey, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
John Malcolm Walker, Institute of Cardiovascular Science, University College London, London, UK; The Hatter Cardiovascular Institute, University College London Hospital, London, UK.
James Moon, Institute of Cardiovascular Science, University College London, London, UK; St Bartholomew’s Hospital, Barts Heart Centre, London EC1A 7BE, UK.
Authors’ contributions
K.D.M., J.M., and J.M.W. contributed to the idea, design of the study, and drafted the manuscript. All authors contributed to data collection, analysis, interpretations, and re-drafting of this manuscript. K.D.M., A.H., and J.M. drafted the study protocol and analysis plan. K.D.M., J.M.W., and J.M. drafted the manuscript and checked data for accuracy of information of the study.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the United Kingdom Foreign & Commonwealth Office, British Embassy in Peru and Cuba, The Peruvian Scientific, Technological Development and Technological Innovation Council (FONDECYT from CONCYTEC Peru), Global Engagement Office, University College London, the National Institute for Health Research University College London Hospitals, the UCLH Charity, and the Maurice Hatter Foundation, and the SCMR.
Conflict of interest
K.D.M. is supported by the Peruvian Scientific, Technological Development and Technological Innovation Council. N.N. has received grants from the South African Medical Research Council, the United Kingdom Medical Research Council, Wellcome Trust, and has received personal fees from Novo Nordisk. Y.H. has received grants, personal fee from the National Institutes of Health, Vertex Inc. and GE and has a patent—US Letter Patent No. 10,638,940. A.H. has a role of leadership at SCMR International Outreach Committee. J.F. has received a personal fee from Siemens AG. K.K. is supported by a British Heart Foundation Clinical Research Training Fellowship. H.L. is supported by the Society of Cardiovascular Magnetic Resonance, has received a grant from Siemens Healthineers, has a Demos Medical Publishing Licence, has honoraria for lectures from Partners Health, receives a payment for expert testimony from Dechert, LLP, has a leadership role at the Radiological Society of North America. M.W. receives consulting fees from Bayer. A.S. is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/18/83/34025). S.S. has a leadership role in the Society for Emergency Radiology, Cardiovascular and Interventional Radiology, and Journal of Vascular and Interventional Radiology. The remaining authors have no relevant conflict of interest.
Data availability
Data used for the current study are all anonymized and are available to other researchers upon reasonable request and approval of the collaborative groups.
References
- 1. Blankstein R. Cardiology patient page. Introduction to noninvasive cardiac imaging. Circulation 2012;125:e267–e271. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3. Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Dehmer GJ. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Thorac Cardiovasc Surg 2019;157:e153–e182. [DOI] [PubMed] [Google Scholar]
- 4. Flett AS, Westwood MA, Davies LC, Mathur A, Moon JC. The prognostic implications of cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2009;2:243–250. [DOI] [PubMed] [Google Scholar]
- 5. Herrey AS, Francis JM, Hughes M, Ntusi NAB. Cardiovascular magnetic resonance can be undertaken in pregnancy and guide clinical decision-making in this patient population. Eur Heart J Cardiovasc Imaging 2019;20:291–297. [DOI] [PubMed] [Google Scholar]
- 6. Abbasi SA, Ertel A, Shah RV, Dandekar V, Chung J, Bhat G, et al. Impact of cardiovascular magnetic resonance on management and clinical decision-making in heart failure patients. J Cardiovasc Magn Reson 2013;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Knobelsdorff-Brenkenhoff F, Schulz-Menger J. Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J Cardiovasc Magn Reson 2015;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganesan AN, Gunton J, Nucifora G, McGavigan AD, Selvanayagam JB. Impact of late gadolinium enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: a systematic review and meta-analysis. Int J Cardiol 2018;254:230–237. [DOI] [PubMed] [Google Scholar]
- 10. Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, et al. On T2* magnetic resonance and cardiac iron. Circulation 2011;123:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagel E, Greenwood JP, McCann GP, Bettencourt N, Shah AM, Hussain ST, et al. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019;380:2418–2428. [DOI] [PubMed] [Google Scholar]
- 14. Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry—multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson 2013;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwong RY, Petersen SE, Schulz-Menger J, Arai AE, Bingham SE, Chen Y, et al. The global cardiovascular magnetic resonance registry (GCMR) of the society for cardiovascular magnetic resonance (SCMR): its goals, rationale, data infrastructure, and current developments. J Cardiovasc Magn Reson 2017;19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Global Health Observatory Data Repository . Magnetic resonance imaging units, per 100 000 population. 2016. [updated 9 March 2016] [Internet]. https://gateway.euro.who.int/en/indicators/hlthres_95-magnetic-resonance-imaging-units-per-100-000/.
- 17. Owolabi M, Miranda JJ, Yaria J, Ovbiagele B. Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Global Health 2016;1:e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdel-Gadir A, Vorasettakarnkij Y, Ngamkasem H, Nordin S, Ako EA, Tumkosit M, et al. Ultrafast magnetic resonance imaging for iron quantification in Thalassemia participants in the developing world: the TIC-TOC study (Thailand and UK International Collaboration in Thalassaemia Optimising Ultrafast CMR). Circulation 2016;134:432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menacho K, Ramirez S, Segura P, Nordin S, Abdel-Gadir A, Illatopa V, et al. INCA (Peru) study: impact of non-invasive cardiac magnetic resonance assessment in the developing world. J Am Heart Assoc 2018;7:e008981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim RJ, Simonetti OP, Westwood M, Kramer CM, Narang A, Friedrich MG, et al. Guidelines for training in cardiovascular magnetic resonance (CMR). J Cardiovasc Magn Reson 2018;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Angelo T, Grigoratos C, Mazziotti S, Bratis K, Pathan F, Blandino A, et al. High-throughput gadobutrol-enhanced CMR: a time and dose optimization study. J Cardiovasc Magn Reson 2017;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shanbhag SM, Greve AM, Aspelund T, Schelbert EB, Cao JJ, Danielsen R, et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur Heart J 2019;40:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong TC, Piehler K, Puntil KS, Moguillansky D, Meier CG, Lacomis JM, et al. Effectiveness of late gadolinium enhancement to improve outcomes prediction in patients referred for cardiovascular magnetic resonance after echocardiography. J Cardiovasc Magn Reson 2013;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keenan NG, Captur G, McCann GP, Berry C, Myerson SG, Fairbairn T, et al. Regional variation in cardiovascular magnetic resonance service delivery across the UK. Heart 2021;107:1974–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nordin S, Kozor R, Medina-Menacho K, Abdel-Gadir A, Baig S, Sado DM, et al. Proposed stages of myocardial phenotype development in fabry disease. JACC Cardiovasc Imaging 2019;12:1673–1683. [DOI] [PubMed] [Google Scholar]
- 26. Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J 2015;36:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han Y, Chen T, Bryant J, Bucciarelli-Ducci C, Dyke C, Elliott MD, et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson 2020;22:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang K, Nakou E, De Garate E, Williams M, Lawton C, Bucciarelli-Ducci C. Implementation of rapid CMR protocol in the COVID-19 era: improving scanning efficiency and increasing scanning capacity. Eur Heart J Cardiovasc Imaging 2021;22, ii22. [Google Scholar]
- 29. Foley JRJ, Richmond C, Fent GJ, Bissell M, Levelt E, Dall’armellina E, et al. Rapid cardiovascular magnetic resonance for ischemic heart disease investigation (RAPID-IHD). JACC Cardiovasc Imaging 2020;13:1632–1634. [DOI] [PubMed] [Google Scholar]
- 30. Kido T, Kido T, Nakamura M, Watanabe K, Schmidt M, Forman C, et al. Compressed sensing real-time cine cardiovascular magnetic resonance: accurate assessment of left ventricular function in a single-breath-hold. J Cardiovasc Magn Reson 2016;18:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for the current study are all anonymized and are available to other researchers upon reasonable request and approval of the collaborative groups.