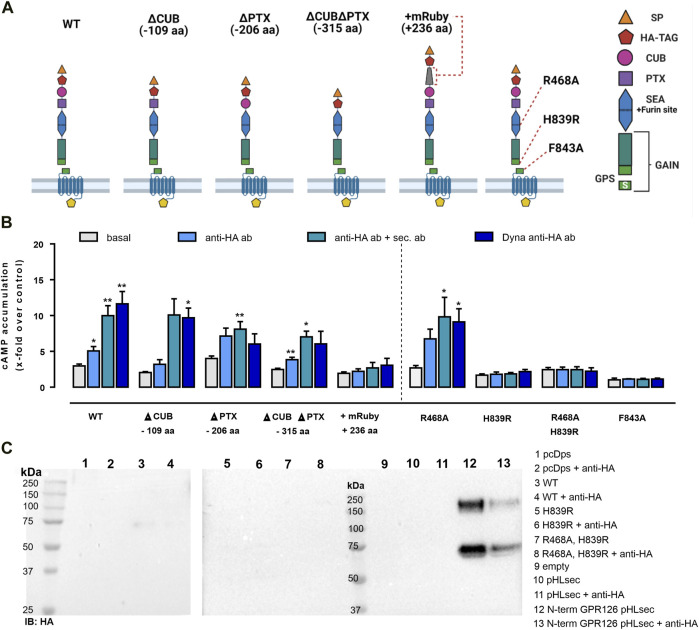
FIGURE 2.
Antibody-mediated activation of GPR126 depends on autoproteolytic cleavage and is obliterated in a construct with an elongated N terminus. (A) Domain architecture of the human GPR126 WT and respective mutants is depicted. The positions of the furin-deficient receptor mutant R468A, the GPS cleavage mutant H839R, and the tethered agonist mutant F843A within the N terminus are displayed. Images were created with BioRender.com. (B) Receptor variants were transfected into COS-7 cells and analyzed with the same antibody-based approach as used in Figure 1 and tested in cAMP assays. An empty vector (pcDps) served as a negative control (cAMP level: 4.3 ± 0.7 nM/well). Data are given as mean ± SEM of three–eight different experiments each performed in triplicates. Statistics were performed by applying one-way ANOVA followed by Dunnett’s post hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001. All shown statistical significance compare the condition to the basal cAMP accumulation of the same construct. Corresponding raw data can be found in the repository. (C) COS-7 cells were transfected with the indicated constructs and treated with 1 µg/ml mouse anti–HA ab for 1 h, 48 h after transfection. Then, supernatants were harvested and analyzed by Western blot. Membranes were incubated with rabbit anti-HA ab and secondary HRP-conjugated anti-rabbit ab. A corresponding Western blot of cell lysates can be found in Supplementary Figure S2C. The secreted N-terminal fragment of GPR126 was only detected when the secretion vector pHLsec containing just the N-terminal fragment of GPR126 as a positive control was transfected.