Abstract
Purpose
With the integration of immunotherapy (IO) agents in the management of metastatic renal cell carcinoma (mRCC), there has been interest in the combined use with radiation therapy (RT). However, real world data are limited. The purpose of this study was to evaluate outcomes in patients with mRCC receiving both RT and IO compared with IO alone.
Methods and Materials
Data were collected from Canadian Kidney Cancer Information System from January 2011 to September 2019 across 14 academic centers. Patients with mRCC who received IO as first- or second-line therapy were included. RT was categorized as radical dose or palliative dose. Kaplan-Meier estimates were reported for overall survival (OS) and time to treatment failure. Cox proportional hazard models were used adjusted for age and International Metastatic RCC Database Consortium risk categories.
Results
In total, 505 patients were included in the study: 179 received RT + IO and 326 received IO alone. Two-year OS for the RT + IO group was 55.0% compared with 66.4% in the IO alone cohort (adjusted hazard ratio [aHR], 1.38; P = .07). At 2 years, 12.2% of the RT + IO patients remained on therapy versus 30.9% in the IO alone group (aHR, 1.30; P = .02). For patients receiving first-line therapy, 2-year OS in the RT + IO group was 56.4% versus 78.4% in the IO alone arm, though this difference was not statistically significant (aHR, 1.23; P = .56). For patients receiving radical dose and palliative dose, 2-year OS was 57.0% and 53.9%, respectively (aHR, 0.86; P = .63).
Conclusions
In this descriptive analysis, more than one-third of patients with mRCC received RT and demonstrated inferior outcomes compared with IO alone. Potential explanations include greater presence of adverse metastatic sites in those receiving RT. Prospective clinical trials evaluating potential benefits of RT in an IO era remain an important need.
Introduction
Over the past decade, the management of metastatic renal cell carcinoma (mRCC) has improved significantly, and increased understanding of the pathways driving disease progression have allowed for rapid development in systemic treatment. With the introduction of immunotherapy (IO) agents, substantial advancements have been made in mRCC, both in the pretreated and untreated settings.1 Nivolumab alone and in combination with ipilimumab have been shown to improve survival and offer durable responses in large randomized trials.2,3 Furthermore, pembrolizumab in combination with either axitinib or lenvatinib and nivolumab combined with cabozantinib have also been approved in the front-line mRCC setting.1,4, 5, 6
Although mRCC was traditionally considered resistant to radiation therapy (RT), there are now data to show this is not the case.7 With improved outcomes and longer survival associated with innovations in modern systemic treatment, RT has increasingly been considered for selected patients with mRCC with oligometastases or oligoprogression on systemic therapy. Furthermore, with the evolution of RT treatment platforms and immobilization techniques facilitating stereotactic body radiation therapy (SBRT), it has been hypothesized that higher doses of radiation in fewer fractions may increase this immunogenic effect while overcoming the innate radioresistance of RCC.8 However, there are not clear guidelines for combining RT and IO in mRCC. There is a need to better understand the relationship between these interventions, particularly regarding optimal patient selection, treatment sequencing, and RT dose-fractionation schedules.
Despite the evolving role of RT in patients receiving IO therapy, real world data remain limited at this time. Several retrospective studies have investigated the use of interleukin-2 (IL-2) and RT but with small sample sizes, and checkpoint inhibitors have only been examined in combination with RT in case reports.9, 10, 11, 12, 13, 14 Our study aimed to describe the outcomes of patients who have received both RT and IO therapy compared with those treated with IO alone using a large prospectively maintained mRCC cohort.
Methods and Materials
Data collection
Data were collected from the Canadian Kidney Cancer Information System, a curated prospective cohort of patients with kidney cancer from 14 academic centers across Canada, shown to be generalizable to the entire kidney cancer population in the country.15 All Canadian Kidney Cancer Information System–participating centers obtained the appropriate local ethics board approval to collect deidentified patient information. Patients included had a confirmed diagnosis of mRCC and received IO as first- or second-line therapy from January 2011 to September 2019 with at least 3 months of follow-up from the date of IO initiation. To be included, RT could be administered up to 1 month before the start of IO or anytime during IO therapy, and these patients were classified as the RT + IO cohort. Patients who died ≤3 months after RT were excluded. Those who received RT were categorized as either having a radical dose (RRT) or palliative dose (PRT) as recorded by the individual centers. RRT was defined as a dose of ≥10 Gray (Gy) for single fraction treatments or ≥5 Gy and/or a biological effective dose of ≥40 Gy for multifraction treatment, assuming an alpha-beta ratio of 10. Dose fractionation schedules that were lower than this were considered palliative. Patients could have multiple courses of RT, with any radical dosing categorizing them into the RRT cohort. Patients who did not receive any RT around the time of IO as described previously were considered the IO alone cohort. Patient demographics, clinical information, and baseline prognostic data were collected. Treatment details including IO timing, RT dose-fractionation, and RT target are summarized.
Endpoints
Overall survival (OS) was defined as the time from IO initiation until death from any cause or a censoring event. Time to treatment failure (TTF) was defined as time from initiation of IO to date of discontinuation of IO, death from any cause, or a censoring event.
Statistical analysis
Descriptive statistics were reported as frequency and proportion for categorical variables. Mean and standard deviation were reported for normally distributed continuous variables while median and interquartile range were reported for nonnormally distributed continuous variables. Kaplan-Meier estimates and the corresponding 95% confidence intervals (CIs) were reported for OS and TTF comparing the RT + IO and the IO alone groups. Log-rank tests were used to compare the Kaplan-Meier curves. Cox proportional hazard models were used for OS and TTF adjusting for age as a continuous variable and International Metastatic RCC Database Consortium (IMDC) risk categories. Adjusted hazard ratio (aHR) and the corresponding 95% CIs were reported. All statistical analyses were conducted using SAS version 9.3 software (SAS Institute Inc, Cary, NC). A P value less than .05 was used for statistical significance.
Results
Patient characteristics and treatment details
A total of 505 patients were included in the study, with a median age of 63 (range, 34-92) (Table 1). The majority of patients were male (77.7%) with clear cell histology (73.7%). Overall, 179 patients received RT + IO, while 326 had IO alone. Karnofsky performance status was balanced between groups, with 81.1% of patients having a Karnofsky performance status score of 80% or higher overall. Patients in the RT + IO group had a greater number of brain (21.8% vs 5.8%; P < .01) and bone metastases (59.2% vs 23.5%; P < .01) compared with those in the IO alone group. A total of 234 patients received first-line combination IO, most commonly ipilimumab and nivolumab, while 271 received second-line monotherapy with nivolumab. There were significant differences in IMDC risk groups and presence of bone and brain metastases between the RT + IO and IO alone cohorts (Table 1, Table EA).
Table 1.
Patient baseline characteristics
| Characteristic, n (%) | Radiation therapy + IO (n = 179) | IO alone (n = 326) | P value |
|---|---|---|---|
| Median age, y (IQR) | 62 (35-88) | 64 (34-92) | .02 |
| Sex | .27 | ||
| Male | 144 (76.2) | 250 (76.2) | |
| Female | 35 (19.6) | 78 (23.8) | |
| IMDC risk group | .03 | ||
| Favorable | 16 (11.3) | 41 (15.0) | |
| Intermediate | 105 (73.9) | 168 (61.3) | |
| Poor | 21 (14.8) | 65 (23.7) | |
| KPS <80% | 21 (12.7) | 42 (13.9) | .72 |
| Line of therapy | .02 | ||
| First-line IO* | 70 (39.1) | 164 (50.3) | |
| Second-line IO | 109 (60.9) | 162 (49.7) | |
| Bone metastases | 106 (59.2) | 77 (23.5) | <.01 |
| Brain metastases | 39 (21.8) | 19 (5.8) | <.01 |
| Nephrectomy | 141 (78.8) | 242 (74.2) | .21 |
| Histology | .90 | ||
| Clear cell | 131 (81.9) | 241 (78.5) | |
| Clear cell papillary | 3 (1.9) | 12 (3.9) | |
| Papillary | 5 (3.1) | 8 (2.6) | |
| Chromophobe | 3 (1.9) | 5 (1.6) | |
| Sarcomatoid | 1 (0.6) | 2 (0.7) | |
| RCC NOS | 11 (6.9) | 19 (6.2) | |
| Other | 6 (3.8) | 20 (6.5) |
Abbreviations: IMDC = International Metastatic RCC Database Consortium; IO = immunotherapy; IQR = interquartile range; KPS = Karnofsky performance status; RCC = renal cell carcinoma; NOS = not otherwise specified
Programmed cell death protein 1 (PD-1) inhibitor + Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitor: 77.6%, PD-1 inhibitor + Vascular endothelial growth factor receptor (VEGF-R) inhibitor: 9.8%, Programmed cell death ligand 1 (PD-L1) inhibitor + VEGF-R inhibitor: 7.3%, PD-L1 inhibitor + VEGF inhibitor: 5.3%.
Within the RT + IO group, 48 patients were categorized as having RRT and 131 as PRT. The most common dose fractionation schedule in the RRT cohort was 30 Gy in 5 fractions (20.8%), and in the PRT group it was 8 Gy in a single fraction (28.2%) (Table 2). The most common sites treated with RT were bone (60.9%) followed by brain (17.3%). In regards to treatment sequence, 25.1% of patients received RT before starting IO, 27.4% had RT within 3 months after IO initiation, and 47.5% had RT greater than 3 months after the IO start date.
Table 2.
Radiation therapy treatment details
| RRT | n (%) | PRT | n (%) | |
|---|---|---|---|---|
| Dose fractionation | ||||
| 30/5 | 10 (20.8) | 8/1 | 37 (28.2) | |
| 20/1 | 7 (14.6) | 20/5 | 36 (27.5) | |
| 24/2 | 6 (12.5) | 30/10 | 25 (19.1) | |
| 12/1 | 6 (12.5) | 12/2 | 6 (4.6) | |
| 21/1 | 5 (10.4) | 17/2 | 3 (2.3) | |
| 35/5 | 4 (8.3) | 15/5 | 3 (2.3) | |
| Other | 10 (20.8) | Other | 21 (16.0) | |
| Sites treated | ||||
| Bone | 19 (39.6) | Bone | 90 (68.7) | |
| Brain | 22 (45.8) | Brain | 9 (6.9) | |
| Lung | 4 (8.3) | Lung | 5 (3.8) | |
| Kidney | 1 (2.1) | Kidney | 4 (3.1) | |
| Other | 2 (4.2) | Other | 23 (17.6) | |
| Timing | ||||
| ≤1 mo before IO initiation | 8 (16.7) | ≤1 mo before IO initiation | 37 (28.2) | |
| ≤3 mo after IO initiation | 10 (20.8) | ≤3 mo after IO initiation | 39 (29.8) | |
| >3 mo after IO initiation | 30 (62.5) | >3 mo after IO initiation | 55 (42.0) |
Abbreviations: IO = immunotherapy; PRT = palliative dose; RRT = radical dose.
Clinical outcomes
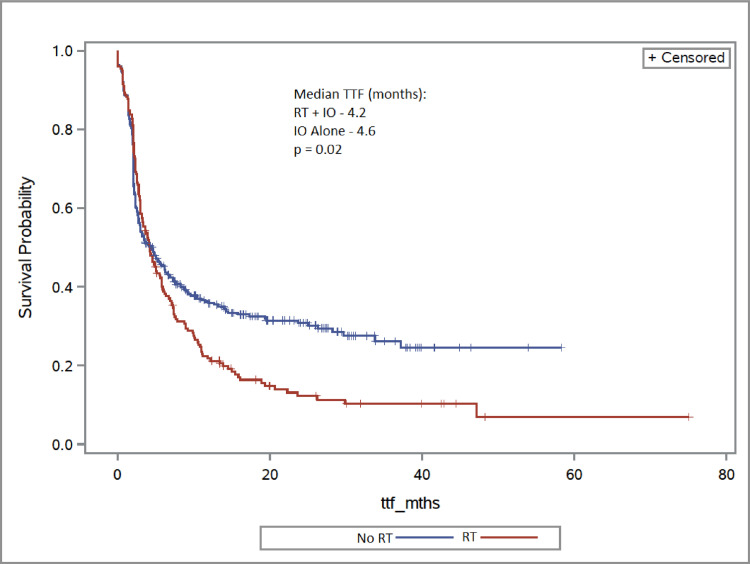
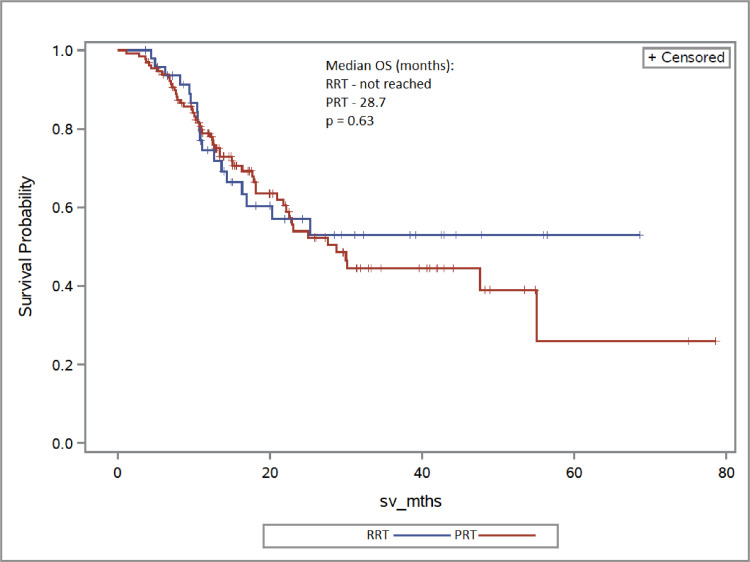
The overall median follow-up was 13.9 months (range, 0.7-78.6). Two-year OS for the RT + IO group was 55.0%, compared with 66.4% in the IO alone cohort (aHR, 1.38; 95% CI, 0.97-1.96; P = .07) (Fig 1). Patients receiving RT + IO had a shorter TTF compared with those who had IO alone, and at 2 years, 12.2% remained on therapy with RT + IO versus 30.9% in the IO alone group (aHR, 1.30; 95% CI, 1.03-1.65; P = .02) (Fig 2). When comparing dose of RT, the RRT cohort had similar survival to the PRT arm, with 2-year OS rates of 57.0% and 53.9%, respectively (aHR, 0.86; 95% CI, 0.47-1.57; P = .63). There was no difference in proportion of patients remaining on treatment at 2 years: 13.5% in the RRT group and 11.9% in the PRT cohort (aHR, 0.99; 95% CI, 0.65-1.50; P = .96) (Fig 3 and 4).
Fig. 1.
Overall survival (OS) of radiation therapy (RT) + immunotherapy (IO) versus IO alone.
Fig. 2.
Time to treatment failure (TTF) of radiation therapy (RT) + immunotherapy (IO) versus IO alone.
Fig. 3.
Overall survival (OS) of radical radiation therapy (RRT) versus palliative radiation therapy (PRT).
Fig. 4.
Time of treatment failure (TTF) of radical radiation therapy (RRT) versus palliative radiation therapy (PRT).
First-line IO
Within the cohort receiving first-line IO, the 2-year OS in the RT + IO group was 56.4% versus 78.4% in the IO alone arm, though this difference was not statistically significant (aHR, 1.23; 95% CI, 0.60-2.53; P = .56). At 2 years, 3.8% of the RT + IO patients remained on treatment, compared with 27.6% in the IO alone group (aHR, 1.48; 95% CI, 1.05-2.10; P = .03). For patients receiving RRT and PRT, 2-year OS was 61.2% and 49.8%, respectively (aHR, 0.54; 95% CI, 0.12-2.33; P = .40), and median TTF was 2.2 months in the RRT group compared with 2.4 months with PRT (aHR, 1.45; 95% CI, 1.01-2.09; P = .04).
In an exploratory analysis of intent of RT, there was no difference in OS comparing RRT (aHR, 0.50; 95% CI, 0.12-2.16; P = .35) or PRT (aHR, 1.60; 95% CI, 0.78-3.25; P = .20) to IO alone (Fig. EB1). TTF also did not differ significantly between the RRT and IO alone groups (aHR, 1.32; 95% CI, 0.74-2.33; P = .34), but the PRT cohort had statistically worse TTF compared with IO alone patients (aHR, 1.45; 95% CI, 1.01-2.09; P = .04) (Fig. EB2).
Second-line IO
In patients who received second-line IO, there was no difference in OS between the RT + IO and IO alone cohorts, with 2-year OS rates of 52.2% and 57.1%, respectively (aHR, 1.28; 95% CI, 0.85-1.92; P = .22). At 2 years, 17.6% of RT + IO patients remained on treatment, compared with 34.5% in the IO alone group, though this was not statistically significant (aHR, 1.27; 95% CI, 0.92-1.75; P = .14).
Discussion
In this real-world analysis of patients with mRCC treated with IO, a significant proportion also received RT. Those who received RT + IO appeared to have worse OS compared with those treated with IO alone; however, this did not reach statistical significance. TTF was worse in the RT + IO group versus IO alone. This finding was also apparent regardless of line of systemic IO therapy.
The outcomes in patients with mRCC also receiving radiation are potentially explained by differences in patient and disease characteristics. The RT + IO group had a greater proportion of patients who had brain and bone metastases, which are known poor prognostic metastatic sites.16, 17, 18 Furthermore, the majority of the RT + IO patients received palliative dose-fractionation regimens with a higher proportion undergoing second-line IO, selecting for those undergoing RT with the primary intent of symptom relief and more likely at a later stage of their disease process. In this study, the receipt of RT was associated with both worsened TTF and OS despite adjustment for IMDC criteria. The need for RT in an unselected setting may be seen as a negative prognostic factor; however, this observation should be tempered given the caveats of sample size and retrospective analysis. Appropriate patient selection, specifically those with oligometastases or oligoprogression, may offer important additional benefits to RT that cannot be defined with granularity from a mixed cohort such as this, despite adjustment for important established prognostic variables.
The current study demonstrated that more than one-third of patients with mRCC treated in this IO era are also receiving RT. This is in keeping with the growing evidence supporting its use in metastatic disease for not only palliation, but also durable local control. The SABR-COMET randomized phase II trial investigated SBRT to up to 5 sites of metastatic disease with a small number of patients with mRCC (2%) included.19 SBRT showed an improvement in OS of 41 months, versus 28 months in the group receiving no ablative therapy, and this was found to be significant within the screening phase II design. Specifically in the mRCC population, SBRT outcomes are encouraging, with Kothari et al20 showing 1-year local control of intra- and extracranial metastases of 88% and 86%, respectively. Similarly, a phase 2 trial by Svedman et al21 demonstrated 98% local control when using SBRT for extracranial disease in primary and metastatic RCC, supporting the use of localized radical treatment especially in settings of otherwise stable systemic disease.
Currently, prospective data combining SBRT with systemic therapy are limited. In an interim analysis of a phase 2 trial analyzing patients with mRCC receiving IL-2, the addition of SBRT had a 2-fold increase in response rates compared with IL-2 alone.22 A Canadian phase 2 trial using SBRT in 37 patients with metastatic RCC with oligoprogression while on sunitinib or pazopanib has been reported.23 At a median follow-up of 11.6 months, the median progression-free survival (PFS) from study entry was 9.6 months, with the vast majority of progression occurring outside of the irradiated areas. The 2-year local control of the irradiated tumors was 96% and the 2-year OS from study entry was 77%. The median time to a change in systemic therapy was 12.6 months, thus prolonging tyrosine kinase inhibitor usage.
Open questions remain regarding the interaction between RT and IO and the potential ability for RT to induce an “abscopal effect.” The optimal dose-fractionation required to potentiate the immune system and leverage a synergistic outcome is not defined, and preclinical evidence has shown wide dose ranges from as low as 12 Gy in a single fraction.24 Furthermore, there is debate on the ideal sequencing of SBRT and IO to best stimulate the immune system. For example, the initiation of IO before RT is mechanistically optimal as it allows repriming of the immune system after checkpoint blockade.25 Conversely, data in mouse models have revealed that adding immunotherapy after completion of RT resulted in significantly higher antitumor immune response.26 In current practice, RT should not be standardly used primarily to induce an abscopal effect in mRCC, and its role in this paradigm should be considered predominantly within confines of prospective studies.
To that end, there are several prospective randomized trials investigating the immunomodulatory effect of RT in combination with IO in mRCC. NIVES is a phase 2 single-arm study looking at SBRT (30 Gy in 3 fractions) to extracranial metastases in combination with nivolumab in mRCC (ClinicalTrials.gov identifier: NCT03469713). Preliminary results have been promising, showing an objective response rate of 26.9% and disease control rate of 58.0%, though this did not meet the primary endpoint.27 RADVAX RCC is evaluating the combination of dual immune checkpoint inhibition plus targeted SBRT (50 Gy in 5 fractions) in patients with mRCC (ClinicalTrials.gov identifier: NCT03065179). The objective response rate was 56% at a median follow-up of 24 months with a median PFS of 8.2 months, and it demonstrated similar toxicity to nivolumab exposure.28 CYTOSHRINK is a phase II randomized trial examining ipilimumab plus nivolumab with or without SBRT to the primary disease using a dose of 30 to 40 Gy in 5 fractions (ClinicalTrials.gov identifier: NCT04090710). The primary endpoint is PFS at 2 years, with secondary outcomes of OS and objective response rate.
Our results should be interpreted in the context of the study design with limitations of a retrospective analysis. There were baseline differences in the populations, particularly in regards to disease distribution, and thus, direct comparisons between cohorts may be confounded. However, outcomes were adjusted for IMDC risk and age, which are otherwise known prognostic factors in mRCC. Another limitation was the categorization of RT treatment intent, and whether patients were treated with the goal of controlling local disease or oligoprogression versus true palliation. Although the indication was characterized using RT treatment data, there may be discrepancies in this classification given the inaccessibility of clinical reports and errors in data coding inherent to the database collection process. In addition, although preclinical data have suggested prescribing higher doses of RT to achieve a sufficient biological effective dose to induce the immune system, the number of patients receiving higher radical doses in this population was comparatively low, limiting a comprehensive analysis of a clinical immunomodulatory effect. Finally, the optimal timing of RT with IO is unknown and the study allowed patients to receive RT up to 1 month before starting immunotherapy to be comprehensive in this exploratory analysis. There are evolving data to suggest that the procytotoxic immune effects of RT may wane by 1 month posttreatment with suppressive changes beginning to predominate.29
Conclusion
This retrospective analysis described the outcomes of a large cohort of patients with mRCC receiving IO with or without RT. OS and TTF of the RT + IO group were less favorable compared with the IO alone cohort, likely due to underlying treatment and disease factors creating a negative selection bias. Given the confounding of the data set and uncertainty regarding the utility of aggressive RT in patients with mRCC, clinicians should be cautious in selecting patients for combined modality therapy based on performance status and metastatic burden. With several prospective trials ongoing, further research should be directed toward case-matched investigations in subgroups of interest, with a focus on RT timing and clarifying appropriate dose-fractionation based on IO agent.
Footnotes
Sources of support: The Kidney Cancer Research Network of Canada and the Canadian Kidney Cancer Information System have received unrestricted grants from Bristol-Myers Squibb, Eisai, EMD Serono, GSK, Ipsen, Pfizer, Merck, Novartis, and Roche. There is no direct role or influence from this funding on this work.
Disclosures: Dr Lalani has received institutional grants from BioCanRx, Bristol-Myers Squibb, Novartis, Roche, Ipsen, and EMD Serono, and honoraria from AbbVie, Astellas, Bayer, Bristol-Myers Squibb, Eisai, Ipsen, Janssen, Merck, Novartis, Pfizer, Roche, and TerSera. Dr Basappa has received consulting fees or honoraria from Merck, Ipsen, Eisai, Roche, Bristol-Myers Squibb, Pfizer, and EMD Serono. Dr Hansen has received institutional grants from GSK, Merck, Pfizer, MedImmune/Genetech, Roche, Janssen, Bristol-Myers Squibb, AstraZeneca, Astellas, Boeringher-Ingelheim, and Bayer, and consulting fees from GSK, Merck, and Eisai. Dr Heng has received consulting fees from Pfizer, Merck, and Bristol-Myers Squibb. Dr Wood has participated on advisory boards for Merck, Astra Zeneca, Bristol-Myers Squibb, and Ipsen. Dr Castonguay has received consulting fees from Pfizer, Bristol-Myers Squibb, Ipsen, and Merck, and honoraria from Pfizer. Dr Soulières has received institutional grants from Bristol-Myers Squibb, Merck, and Pfizer, and honoraria from Merck. Dr Winquist has received consulting fees from Merck. Dr Canil has received honoraria from Janssen, Bayer, Eisai, Astellas, Merck, Rosche, Bristol-Myers Squibb, Ipsen, Pfizer, Amgen, Ferring, and Seattle Genetics; conference support from Sanofi-Genzyme and Janssen; and institutional payments from Eisai and Pfizer. Dr Graham has received consulting fees from Ipsen, Pfizer, and Janssen, and honoraria from Ipsen and Pfizer. Dr Bjarnason has received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Eisai, and Ipsen. Dr Pouliot has participated on advisory boards for Esai and Merck. Dr Swaminath has received honoraria from AstraZeneca, Bristol-Myers Squibb, and Eisai. All other authors have no disclosures to declare.
This study was based on the Canadian Kidney Cancer Information System data. The authors do not own these data and hence are not permitted to share them in the original form (only in aggregate form, eg, publications). All cancer registrations are owned and maintained by the Kidney Cancer Research Network of Canada.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022. 100899.
Appendix. Supplementary materials
References
- 1.Lalani A-KA, McGregor BA, Albiges L, et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: Current paradigms, use of immunotherapy, and future Directions. Eur Urol. 2019;75:100–110. doi: 10.1016/j.eururo.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer R, Alekseev B, Rha S-Y, et al. Lenvatinib plus Pembrolizumab or Everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 7.Siva S, Kothari G, Muacevic A, et al. Radiotherapy for renal cell carcinoma: Renaissance of an overlooked approach. Nat Rev Urol. 2017;14:549–563. doi: 10.1038/nrurol.2017.87. [DOI] [PubMed] [Google Scholar]
- 8.Buttigliero C, Allis S, Tucci M, et al. Role of radiotherapy in improving activity of immune-modulating drugs in advanced renal cancer: Biological rationale and clinical evidences. Cancer Treat Rev. 2018;69:215–223. doi: 10.1016/j.ctrv.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Lange JR, Raubitschek AA, Pockaj BA, et al. A pilot study of the combination of interleukin-2-based immunotherapy and radiation therapy. J Immunother. 1992:12. doi: 10.1097/00002371-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2: Tumor and immunological responses. Sci Transl Med. 2012;4:1–8. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 11.Takada T, Yamada Y, Uno M, Komeda H, Fujimoto Y. [Synergetic responses after administration of interleukin-2 and Interferon-alpha combined with gamma knife radiosurgery in a patient with multiple lung and brain metastases: a case report] Hinyokika Kiyo. 2005;51:381–384. [PubMed] [Google Scholar]
- 12.Dengina N, Mitin T, Gamayunov S, Safina S, Kreinina Y, Tsimafeyeu I. Stereotactic body radiation therapy in combination with systemic therapy for metastatic renal cell carcinoma: A prospective multicentre study. ESMO Open. 2019;4:1–6. doi: 10.1136/esmoopen-2019-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie G, Gu D, Zhang L, Chen S, Wu D. A rapid and systemic complete response to stereotactic body radiation therapy and pembrolizumab in a patient with metastatic renal cell carcinoma. Cancer Biol Ther. 2017;18:547–551. doi: 10.1080/15384047.2017.1345389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaPlant Q, Deselm C, Lockney NA, Hsieh J, Yamada Y. Potential abscopal response to dual checkpoint blockade in RCC after reirradiation using dose-painting SBRT. Pract Radiat Oncol. 2017;7:396–399. doi: 10.1016/j.prro.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Tajzler C, Tanguay S, Mallick R, et al. Determining generalizability of the Canadian Kidney Cancer information system (CKCis) to the entire Canadian kidney cancer population. Can Urol Assoc J. 2020;14:499–506. doi: 10.5489/cuaj.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S-C, Kuo P-L. Bone metastasis from renal cell carcinoma. Int J Mol Sci. 2016;17:987. doi: 10.3390/ijms17060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodward E, Jagdev S, McParland L, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48:160–166. doi: 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Shuch B, La Rochelle JC, Klatte T, et al. Brain metastasis from renal cell carcinoma: Presentation, recurrence, and survival. Cancer. 2008;113:1641–1648. doi: 10.1002/cncr.23769. [DOI] [PubMed] [Google Scholar]
- 19.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 20.Kothari G, Foroudi F, Gill S, Corcoran NM, Siva S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol. 2015;54:148–157. doi: 10.3109/0284186X.2014.939298. [DOI] [PubMed] [Google Scholar]
- 21.Svedman C, Sandström P, Pisa P, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 22.Hannan R, Ishihara D, Louder K, et al. Phase II trial of high-dose interleukin-2 (IL-2) and stereotactic radiation therapy (SABR) for metastatic clear cell renal cell carcinoma (ccRCC): Interim analysis. J Clin Oncol. 2016;34(2 suppl):532. [Google Scholar]
- 23.Cheung P, Patel S, North SA, et al. A phase II multicenter study of stereotactic radiotherapy (SRT) for oligoprogression in metastatic renal cell cancer (mRCC) patients receiving tyrosine kinase inhibitor (TKI) therapy. J Clin Oncol. 2020;38(15 suppl):5065. [Google Scholar]
- 24.Deng L, Weichselbaum RR, Fu Y, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Gan L, Na A, Ge L, Chen B, Liu J. Combination with stereotactic body radiotherapy offers a promising strategy to overcome resistance to immunotherapy in advanced renal cell cancer. J Oncol. 2019 doi: 10.1155/2019/1483406. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masini C, Iotti C, Giorgi U, et al. Nivolumab (NIVO) in combination with stereotactic body radiotherapy (SBRT) in pretreated patients (pts) with metastatic renal cell carcinoma (mRCC): First results of phase II NIVES study. J Clin Oncol. 2020;38:613. [Google Scholar]
- 28.Hammers HJ, Vonmerveldt D, Ahn C, et al. Combination of dual immune checkpoint inhibition (ICI) with stereotactic radiation (SBRT) in metastatic renal cell carcinoma (mRCC) (RADVAX RCC) J Clin Oncol. 2020;38(6 suppl):614. [Google Scholar]
- 29.Chow J, Hoffend NC, Abrams SI, Schwaab T, Singh AK, Muhitch JB. Radiation induces dynamic changes to the T cell repertoire in renal cell carcinoma patients. Proc Natl Acad Sci U S A. 2020;117:23721–23729. doi: 10.1073/pnas.2001933117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.