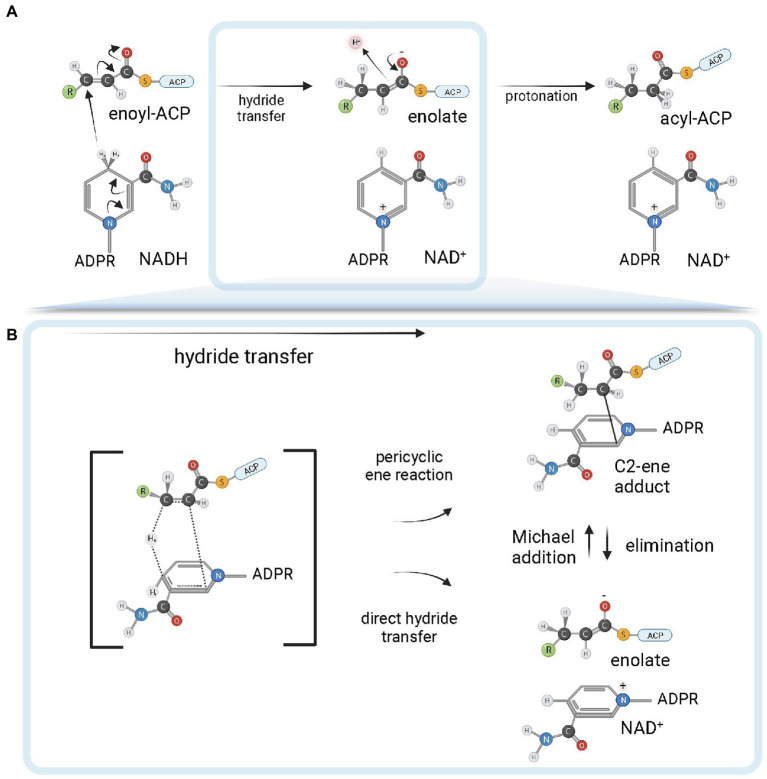
Figure 4.
FabI reaction mechanism. (A) Reduction of trans-2-acyl-ACP (enoyl-ACP) to acyl-ACP by FabI enzymes. A reduced form of the dinucleotide cofactor (NADH or NADPH, depending on the enzyme) serves as the reductant species. In the first half-reaction, bacterial FabI enzymes catalyze the hydride transfer from the 4S hydrogen position of the nicotinamide ring (represented in the figure) of the cofactor to the C3 position (Cβ) of the α,β-unsaturated thioester of the enoyl-ACP substrate. An enolate intermediate is formed with the concomitant oxidation of NADH to NAD+. In the second half-reaction, the enolate intermediate is protonated, leading to the formation of the acyl-ACP product. (B) Covalent ene adduct intermediate between NADH and enoyl-ACP substrate. In this covalent intermediate species, there is a covalent bond between the C2 atom of the NADH nicotinamide ring and the C2 atom of the acyl substrate. Two alternate possibilities for hydride transfer are depicted. The hydride transfer could be the result of a pericyclic ene reaction from a transition state that leads to the C2-ene adduct or could be the result of a direct hydride transfer. In the latter, C2-ene adduct would be formed by Michael addition from the intermediate enolate and NAD+. Created with BioRender.com.