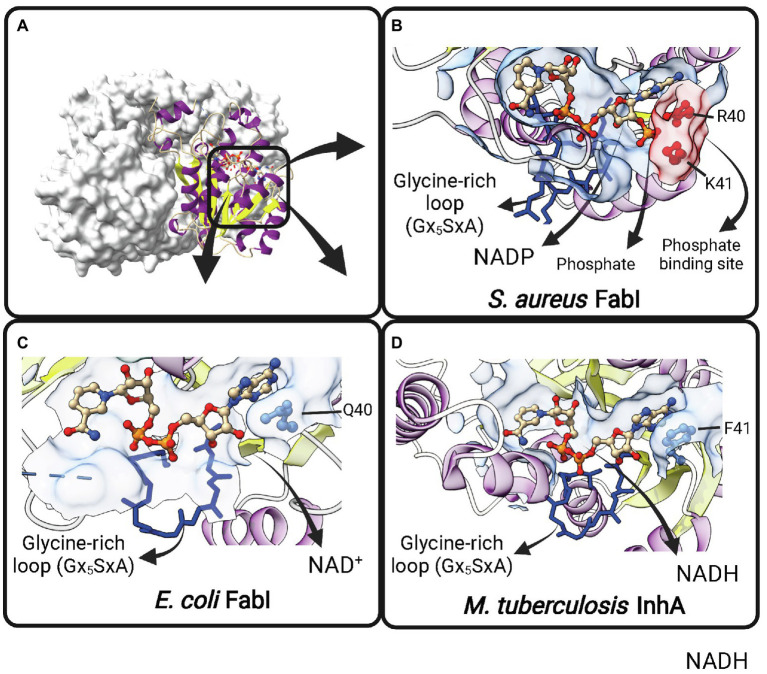
Figure 6.
Cofactor-binding pockets of ENR FabIs. (A) Tetrameric structure of E. coli FabI (EcFabI, PDB: 1DFI) as a prototypical tetrameric ENR FabI. Three protomers are represented in surface view and the fourth in cartoon representation. (B) Cofactor-binding site of Staphylococcus aureus FabI (SaFabI) bound to NADP (PDB: 3GR6). The positively charged R40 and K41 residues (in red) that interact with the phosphate bound to the adenosine ribose of NADP are highlighted. (C) Cofactor-binding site of EcFabI bound to NAD+ (PDB: 1DFI). The polar residue Q40 is found in the place of the positively charged residues required to accommodate the additional phosphate of NADP. (D) Cofactor-binding site of Mycobacterium tuberculosis InhA bound to NADH (PDB: 1ENY). The nonpolar F41 is found in the same approximate position as Q40 in EcFabI and K41 and R40 in SaFabI. (B–D) The Glycine-rich loop is highlighted in dark blue. Created with UCSF ChimeraX (Pettersen et al., 2021) and BioRender.com.