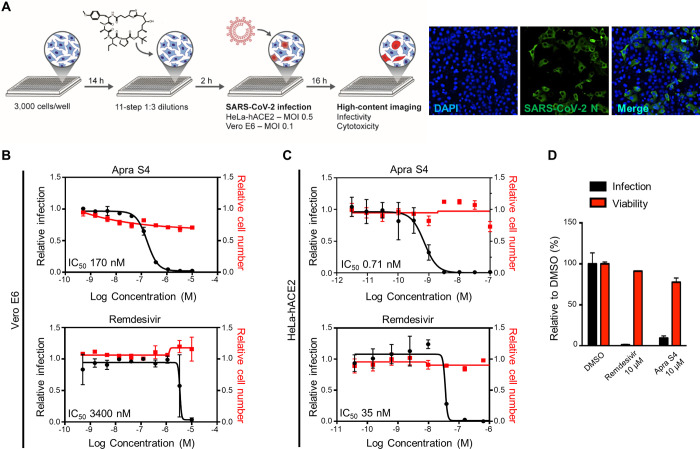
Figure 2.
Screening approach and activities of Apra S4 against SARS-CoV-2 compared with remdesivir. (A) Three thousand cells per well were seeded in a 384-well black plate 16 h prior to infection. Two hours prior to infection, cells were treated with remdesivir or Apra S4 in 11-step 1:3 dilutions at indicated starting concentrations, in triplicate. SARS-CoV-2 USA-WA1/2020 was added to each well, at an MOI = 0.1 (Vero E6) or 1 (HeLa-hACE2). Sixteen hours postinfection, cells were fixed and analyzed by immunofluorescence imaging. For each condition, the percentage of infection was calculated as the ratio of the number of infected cells stained for coronavirus N to number of cells stained with DAPI. (B,C) Dose–response analysis for Vero E6 (B) and HeLa-hACE2 cells (C). (D) Antiviral activity of Apra S4 against SARS-CoV-2 infection in human stem cell derived pneumocyte-like cells. Human stem cell derived pneumocyte-like cells were treated with remdesivir or Apra S4 (10 μM) for 1 h at 37 °C followed by SARS-CoV-2 USA-WA1/2020 infection for 48 h at 37 °C. Then, the cells were dissociated using cell dissociation buffer and fixed in 4% methanol-free formaldehyde for the following FACS analysis. The fixed cells were incubated with antimouse SARS-CoV-2 N protein antibody for 1 h and the infected cells were counted by flow cytometry. The data were represented for each triplicate as the percentage of cells infected compared to the DMSO control. The duplicate set of cells were treated with same drug concentrations but left uninfected. After 48 h of incubation at 37 °C, the cells were analyzed for viability using an MTT assay.