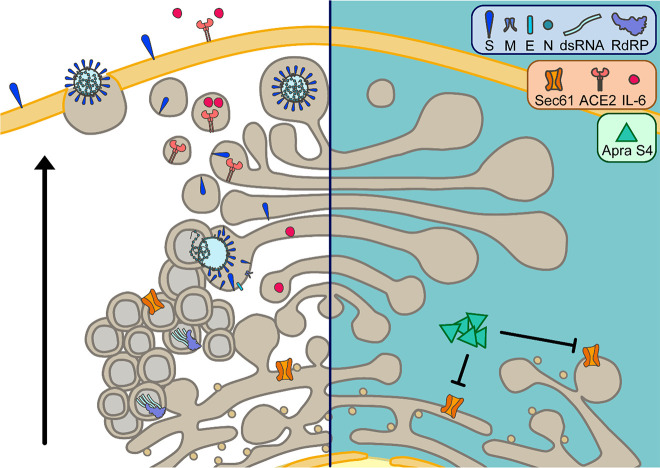
Figure 7.
Putative antiviral action and effects on host proteins induced by apratoxin treatment as a consequence of Sec61 inhibition (left: uninhibited state, right: inhibited state). Apratoxins, like Apra S4, potently inhibit Sec61α, a subunit of the Sec61-translocon in the ER membrane. Sec61 inhibition by Apra S4 reduces the SARS-CoV-2-induced formation of double-membrane vesicles, the site of viral genome replication. Consequently, replication intermediates such as double-stranded RNA are strongly reduced upon Apra S4 treatment. In addition, expression of viral proteins is negatively affected through Apra S4-mediated inhibition of Sec61. Especially expression of structural proteins that require cotranslational translocation into the ER, presumably in a Sec61-dependent fashion, to undergo N-glycosylation and other posttranslational modifications is prevented by Sec61 inhibition. As a consequence, virus assembly and budding in the ERGIC (ER-Golgi intermediate compartment), trafficking, and release from cells via exocytosis is abrogated in Apra S4-treated cells. Furthermore, apratoxin treatment affects expression of cellular proteins, which require Sec61-mediated cotranslational translocation. Among these proteins, downregulation of ACE2 expression and inhibition of cytokine secretion could further affect SARS-CoV-2 infection and disease progression.