FIGURE 1.
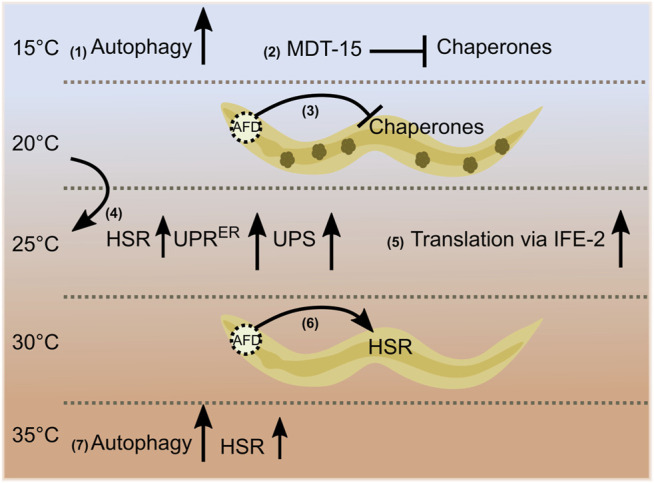
Temperature-dependent regulation of proteostasis. Autophagy and chaperone levels are affected at low temperatures via fatty acid signaling (1), (2). A wild-type thermosensory circuit attenuates protein folding during chronic stress; small dark green structures inside the worm indicate protein aggregates (3). A 1-day transfer of late larval staged C. elegans from 20 to 25°C increases the heat shock response [HSR (mildly, small arrow)], the unfolded protein response in the endoplasmic reticulum (UPRER), and the activity of the ubiquitin/proteasome-system (UPS) in the intestine (4). The translational efficiency of selective mRNAs (msh-4/him-14, msh-5) increases at 25°C via an eIF4E family protein, IFE-2 (5). AFD thermosensory neuron cells non-autonomously regulate the heat shock response [acute heat shock (HS) of 30°C for 15 min] (6). Hormetic heat shock of 1 h at 36°C induces autophagy and selective HSR (7).
