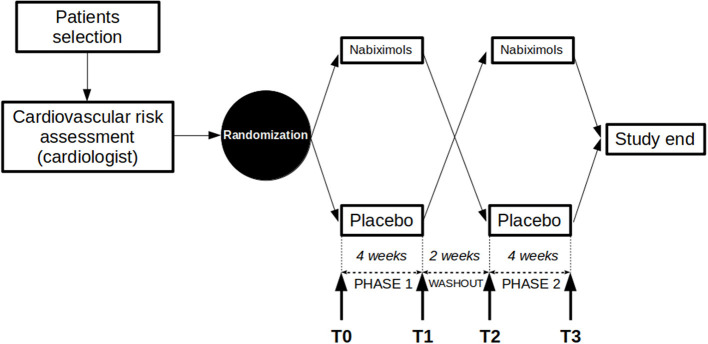
Figure 1.
Study protocol design. Study crossover design is represented. After checking for inclusion/exclusion criteria and passing the cardiological evaluation, patients were randomized to placebo/nabiximols or nabiximols/placebo arms. The two phases of the study were separated by a washout period, started with visits T0/T2 and ended with visits T1/T3.