Abstract
Preeclampsia (PE) is a common pregnancy-specific disorder that is a major cause of both maternal and fetal morbidity and mortality. Central to the pathogenesis of PE is the production of antiangiogenic and inflammatory factors by the hypoxic placenta, leading to the downstream manifestations of the disease, including hypertension and end-organ damage. Currently, effective treatments are limited for PE; however, the development of preclinical animal models has helped in the development and evaluation of new therapeutics.
In this review, we will summarize some of the more commonly used models of PE and highlight their similarities to the human syndrome, as well as the therapeutics tested in each model.
Keywords: preeclampsia, pregnancy, animal models, hypertension
Preeclampsia (PE) is a highly variable syndrome of pregnancy that is defined by new-onset hypertension (HTN) after 20 weeks of gestation as well as one or more of the following criteria: proteinuria, thrombocytopenia, pulmonary edema, renal insufficiency, liver impairment, or visual or cerebral disturbances (1). PE affects roughly 5% to 7% of all pregnancies and is a leading cause of maternal and fetal mortality (2). Furthermore, PE leads to long-term health consequences both for the mother and her offspring, including increased risk for cardiovascular disease and metabolic syndrome (3). An increasing abundance of clinical data support the concept that there is more than one subtype of PE, but a consensus on the number of subtypes or the best way to categorize them is lacking (4). Most commonly, PE is divided into early-onset PE (< 34 weeks of gestation), which tends to be more severe and associated with intrauterine growth restriction (IUGR) (5), and late-onset (> 34 weeks of gestation), which is usually milder (4). Early-onset PE is thought to be the result of defective placentation, whereas late onset is more likely the result of placental senescence and maternal comorbidities such as cardiovascular and metabolic diseases (6). An additional type of PE, superimposed, occurs in women with preexisting chronic HTN. Roughly 20% of pregnant women with chronic HTN develop PE, and it is diagnosed based on the presence of additional symptoms such as thrombocytopenia, liver dysfunction, or other symptoms indicative of the disease (7).
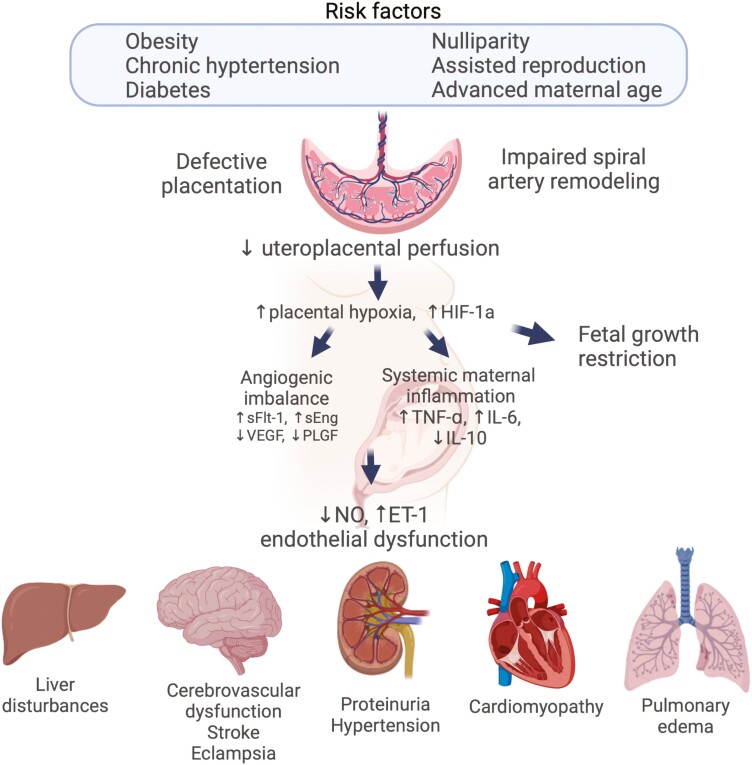
In a healthy pregnancy, fetal cytotrophoblast cells invade the spiral arteries of the uterus, in order to increase the size of these vessels to allow for adequate blood supply for the developing fetus. However, in many cases of PE, spiral artery remodeling is impaired, resulting in inadequate blood flow and the development of placental ischemia. The ischemic placenta leads to increases in oxidative stress and the release of various inflammatory cytokines, antiangiogenic factors, and vasoactive substances, including tumor necrosis factor-α (TNF-α), soluble fms-like tyrosine kinase-1 (sFlt-1), and endothelin-1 (ET-1) that lead to widespread endothelial dysfunction, leading to the downstream manifestations of the disease (Fig. 1) (8). Risk factors for PE include advanced maternal age, nulliparity, obesity, chronic HTN, and the use of assisted reproductive technology (2, 9), all of which are increasing.
Figure 1.
Schematic of pathways leading to the development of preeclampsia. Created with Biorender.com.
Currently, therapeutic options for the treatment of PE are limited to prolonging gestation and treatment with anticonvulsives to prevent seizures, as delivery of the placenta and fetus is the only way to cure the disorder (10). However, because the rates of PE continue to rise worldwide, considerable effort has been made to fully elucidate mechanisms of disease pathogenesis and to develop therapeutics that are safe both for mother and baby. Various animal models have been developed over the past several decades to study the manifestations of the disease and identify potential therapeutic targets. Broadly speaking, these models include immune-mediated, genetic, pharmacological/exogenous agent administration, and surgical manipulation. Herein, we will discuss many of these models, their similarities to the human syndrome, and the therapeutics tested in each model. A summary of the models and the therapeutics tested is also outlined in Table 1.
Table 1.
Summary of animal models of preeclampsia
| Model | HTN | Proteinuria | Endothelial dysfunction | IUGR | Angiogenic imbalance | Additional phenotypes | Therapeutics tested | References | |
|---|---|---|---|---|---|---|---|---|---|
| Immune mediated | Low-dose LPS | ✓ | ✓ | ? | ✓ | ✓ | ↑ renal injury, ↑ fetal resorptions | Low-dose aspirin, quercitin, lipoxin A4, curcumin, metformin, nicotine, galectin 9 | (11-22) |
| TLR 3, 7, and 8 stimulation | ✓ | ✓ | ✓ | ✗ | ? | ↑ fetal demise, placental inflammation, systemic inflammation | IL-4 and IL-10 cosupplementation, MHC class II invariant chain depletion, γ δ T-cell depletion | (23-26) | |
| TLR9 stimulation | ✓ | ✓ | ✗ | ✗ | ✓ | Excess vasoconstriction and oxidative stress, placental inflammation, ↑ fetal resorptions | Low-dose aspirin | (27, 28) | |
| C1q –/– mouse | ✓ | ✓ | ✓ | ✓ | ✓ | ↑endotheliosis, ↑fetal death | Pravastatin, L-citrulline | (29–31) | |
| TNF-α infusion | ✓ | ✓ | ✓ | ✗ | Mild | ↓ GFR, ↓ renal plasma flow, ↑ HIF-1α | Endothelin A receptor antagonist, HO-1 induction | (32–37) | |
| IL-10 –/– mouse | ✓ | ✓ | ✓ | ✗ | ? | Placental inflammation, systemic inflammation | (38) | ||
| IL-4 –/– mouse | ✓ | ✓ | ✓ | ✗ | ? | Placental inflammation, systemic inflammation | (39) | ||
| Spontaneous/genetic | BPH/5 mouse | ✓ | ✓ | ✓ | ✓ | ✓ | Abnormal spiral artery remodeling, complement activation at maternal/fetal interface, hyperleptinemia | Tempol, Cox2 inhibition, complement inhibition | (40-43) |
| Dahl S rat | ✓ | ✓ | ✓ | ✓ | ✓ | ↑ uterine artery resistance, glomerulomegaly, placental hypoxia | PDE5 inhibition, sodium thiosulfate, L-citrulline, 1,3-butanediol | (44-49) | |
| Surgical/pharmacological | L-NAME infusion | ✓ | ✓ | ✓ | ✓ | ✓ | ↓ placental weight | L-arginine, PDE5 inhibition, vagus nerve stimulation, VEGF, fibroblast growth factor type 2 | (50–59) |
| Arginine vasopressin | ✓ | Mild | ✗ | ✓ | ✗ | Impaired spiral artery remodeling | None | (60, 61) | |
| sFlt-1 overexpression/infusion | ✓ | ✓ | ✓ | ✓ | ✓ | ↓ placental weight, ↑ ET-1, ↑ ROS | Pravastatin, HO-induction, VEGF | (62-66) | |
| Uterine artery ligation | ✓ | ✓ | ? | ? | ✓ | PlGF, sFlt-1 siRNA | (67-69) | ||
| Reduced uterine perfusion pressure | ✓ | ✓ | ✓ | ✓ | ✓ | ↑ peripheral vascular resistance, ↑ ET-1, ↓NO, ↓ GFR, ↓ RPF, ↑ inflammatory cytokines, ↑ BBB permeability | Endothelin type A receptor blockade, L-arginine, TNF-ɑ blockade, tempol, HO-1 induction, L-ergothioneine, VEGF, pravastatin, PlGF, nicotine, neutrophil depletion, complement inhibition | (66, 70-81) |
Abbreviations: ✓, present; ✗, absent; ?, unknown or not tested; BBB, blood-brain barrier; Dahl S, Dahl salt-sensitive; ET-1, endothelin-1; GFR, glomerular filtration rate; HTN, hypertension; IL, interleukin; IUGR, intrauterine growth restriction; L-NAME, N-nitro-L-arginine methyl ester; LPS, lipopolysaccharide; NO, nitric oxide; PDE5, phosphodiesterase 5; PlGF, placental growth factor; ROS, reactive oxygen species; RPF, renal plasma flow; siRNA, small interfering RNA; sFlt-1, soluble fms-like tyrosine kinase-1; TLR, toll-like receptor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Immune-Mediated Models of Preeclampsia
A successful pregnancy requires well-timed immunomodulation to facilitate successful implantation, placentation, and delivery. Medawar published his theories of maternal-fetal tolerance in 1953, and while none of his theories have proven correct, they nonetheless provided a basis to understand the tolerance of the maternal immune system to the semiallogeneic fetus (82). As fetal trophoblasts begin to invade the endometrial tissue, various immune cells in the decidua express activation markers, suggesting that they recognize and respond to the trophoblasts (83). However, several mechanisms are at play to maintain tolerance to the fetus. CD4 + CD25 + FoxP3 + regulatory T cells (Tregs) are essential in maintaining pregnancy (84), and specific paternal antigen-specific T cells have been identified both systemically and in the placenta in mice (85). The same occurs in human pregnancy, although paternal antigen-specific Tregs have not been identified (86). Invading fetal trophoblasts also protect themselves by producing suppressive cytokines and expressing nonpolymorphic major histocompatibility complex (MHC) molecules that are recognized by maternal T and natural killer (NK) cells (87-90). Sargent et al (91) proposed that an abnormal recognition of fetal trophoblast cells by maternal decidual NK cells leads to inadequate trophoblast invasion and a defect in the remodeling of spiral arteries. This leads to an ischemic placenta and a maternal inflammatory response that causes the clinical manifestations of PE. Many other immune changes have been observed both in the placenta and in the circulation of preeclamptic pregnancies. Briefly, this is characterized by a more “inflammatory” phenotype in PE with excessive complement activation and T-helper subset dysregulation. In addition, the release of danger-associated molecular patterns (DAMPs) that bind to toll-like receptors (TLRs) and other pattern recognition receptors as a result of poor placentation and oxidative stress can lead to the downstream symptoms of PE (92). Several animal models have been developed or discovered that support each of these immunological changes that can lead to the development of PE.
Danger-associated Molecular Pattern Models
Various clinical studies have linked urinary tract infections (93), periodontal disease (94), COVID-19 infection (95), and other primary viral infections (96, 97) to the development of PE, which suggests that various DAMPs that are released during infection may play a role in PE pathogenesis. DAMPs bind to innate immune receptors, including TLRs, nucleotide-binding oligomerization domain-like receptors, and scavenger receptors. TLRs 1 to 9 are expressed at the maternal-fetal interface and are important for establishing an optimal environment for implantation and placentation (98, 99). Because of the clinical association of infections with the development of PE, various models have been developed that rely on stimulation of TLRs to elicit a PE-like syndrome in rodents.
Low-dose lipopolysaccharide
Several models have been described that involve treatment of pregnant rats with a low dose of lipopolysaccharide (LPS) from gram-negative bacteria (11-15). LPS binds to a cell surface complex containing CD14, TLR4, and MD2 and triggers downstream signaling to recruit the transcription factor nuclear factor (NF)κB to the nucleus, leading to the transcription of proinflammatory cytokines. Most studies report the use of LPS from Escherichia coli serotype O111:B4; however, there are differences in the timing, route, and dose of LPS used. Faas et al (11) was the first to use this model, and gave a single dose of 1.0 mg/kg via jugular vein infusion to pregnant Wistar rats at gestational day (GD)14. Increased blood pressure (BP), albuminuria, and renal inflammation were demonstrated in this model (11, 12). Similarly, Cotechini and colleagues (14) treated rats via intraperitoneal injection daily from GD13.5 to GD16.5, which led to persistent systemic inflammatory response, characterized by increased circulating TNF-α and leukocytes and increased numbers of activated macrophages in the placentas. In addition, rats given LPS had deficient trophoblast invasion and insufficient remodeling of the uterine spiral arteries. It is likely that the increased number of macrophages in the placenta secrete increased TNF-α, which can impair trophoblast migration and affect spiral artery remodeling (100, 101). The pups born to LPS-treated dams also had lower fetal weight. BP, as assessed using telemetry, was marginally higher in pregnant rats given LPS as compared to pregnant rats given saline. In addition, BP did not drop in late pregnancy in pregnant LPS-treated rats as it does in a normal pregnant animal. The maternal inflammatory response as a result of LPS was mediated by TNF-α (14). In an additional LPS model, a single dose of LPS (0.5 mg/kg) was given to pregnant Sprague-Dawley rats at GD5 via tail vein infusion (15). GD5 is immediately post implantation and corresponds to early placentation in rats (102). This single dose of LPS was sufficient to increase BP and urinary protein excretion throughout the remainder of the pregnancy. The rats that received LPS also had increased fetal resorptions and fetal growth restriction. TLR4 and NFκB expression were increased in the placentas of rats that received LPS (15). Curcumin was used as an intervention and was found to improve outcomes in LPS-treated rats via a suppression of TLR4 signaling (16). Because LPS induces systemic inflammation, some of the phenotypic changes are not pregnancy specific. However, administration of the same dose of LPS to nonpregnant animals did not lead to changes in BP in the single-dose model (15), and increased BP to a lesser extent compared to pregnant rats in the model in which the treatments are from GD13.5 to GD16.5 (14).
Toll-like receptor 3, 7, and 8 agonists
An additional DAMP-induced model of PE involves the stimulation of the TLRs 3, 7, or 8, which are TLRs that are expressed on endosomal membranes. TLR3 binds to double-stranded DNA, whereas TLRs 7 and 8 bind single-stranded RNA. These TLRs are expressed by immune cells, vascular endothelial cells, and also by various cells in the placenta. In addition to binding to virally derived nucleic acids, they can also bind to RNA released from apoptotic and necrotic cells or endogenous messenger RNA (103, 104). Women with PE were shown to have increased expression of TLR3, 7, and 8 in the placenta as compared to normal pregnant women in a small clinical cohort (23). An initial study determined that TLR3 activation during pregnancy in rats induced endothelium dysfunction and increased BP (24). In a subsequent study in mice, pregnant mice were treated with the TLR3-specific agonist Poly I:C, the TLR7 agonist R-837, or the TLR7/8 agonist CLO97 via intraperitoneal injection on GD13, 15, and 17. Animals given the TLR agonists had increased BP, as measured by tail-cuff plethysmography at GD17, and impaired endothelium-dependent relaxation in aortic rings. The mice also had increased urinary protein excretion and increased fetal demise (23). Cotreatment of pregnant dams given polyI:C with interleukin (IL)-4 and IL-10 lowered BP, improved endothelial function, and prevented proteinuria (25).
Toll-like receptor 9 agonist
TLR9, similar to TLRs 3,7, and 8, is a nucleic acid sensing TLR that recognizes hypomethylated CpG motifs that are common in bacterial, mitochondrial, and fetal DNA (105). Several groups have tested the effects of TLR9 stimulation during pregnancy. Goulopoulou et al (27) treated rats with the synthetic oligodeoxynucleotide ODN2395 containing CpG motifs on GD14, 17, and 18. This treatment induced HTN in pregnant dams and led to excess vasoconstriction and oxidative stress in mesenteric resistance arteries. In an additional study, mice were treated with ODN 1826, which also caused PE symptoms including HTN and proteinuria. The authors also observed increased inflammation in the placenta as well as angiogenic imbalance, evidenced by decreased vascular endothelial growth factor (VEGF) and increased sFlt-1 expression in placental tissue (106). Low-dose aspirin treatment ameliorated the symptoms of PE in rats treated with the TLR9 agonist (28).
It is important to note that administration of the same doses of these TLR stimuli discussed to nonpregnant animals does not cause HTN, vascular function, or other symptoms indicative of PE, suggesting that the underlying inflammatory state of pregnancy contributes to the effects of these stimuli. Unresolved questions include the cell types that mediate the responses to the TLRs, and whether abnormal TLR stimulation is a causative factor in the development of PE.
C1q–/– Mouse
The complement system plays dual roles in a healthy pregnancy; complement inhibitors protect the growing fetus at the maternal/fetal interface, while activation of complement is also important for implantation, development of the fetus, and labor (107). A genetic analysis of the C3 gene found single-nucleotide variations that were associated with the development of severe PE (108). The complement component C1q, in complex with C1r and C1s, recognizes antigen:antibody complexes to activate the classical pathway of complement. C1q is also synthesized and secreted by fetal cytotrophoblasts and binds to extracellular matrix proteins to mediate adhesion and migration within the decidua (109). Singh et al (29) mated C1q–/– male mice to C1q–/– female mice, which led to features of PE, including defective placentation, HTN, renal injury, vascular dysfunction, and angiogenic imbalance. Interestingly, pregnancies in which a C57BL/6J female mouse is mated with a male C1q–/– mouse result in a similar phenotype reported by 2 research groups (29, 30). Notably, both groups report HTN, fetal growth restriction, renal injury, and vascular dysfunction; however, the report by Sutton et al (30) did not show angiogenic imbalance. The reason for this is likely because the placenta is more influenced by paternal genes, which promote fetal growth (110). Because the male lacks C1q, this would impair trophoblast invasion and spiral artery remodeling. The mothers were followed post partum, and despite a drop in BP after delivery, the mice had an elevated BP compared to mice mated with C57BL/6 up to 60 days after delivery (29). Vascular dysfunction persists for up to 7 months after delivery (30). Interestingly, the authors note that the data suggest the arteries have adapted to rely less on nitric oxide (NO) for relaxation, as the vessels have a significant improvement in relaxation in the presence of N-nitro-L-arginine methyl ester (L-NAME), despite minor impairments in relaxation persisting. A similar decrease in NO contribution to dilation in resistance arteries was also shown post partum in a rat model of placental ischemia (111). Several therapeutics have been tested in this model; pravastatin was shown to prevent PE when given during pregnancy (29). L-citrulline, a nonessential amino acid that is converted to L-arginine and promotes NO production, was recently given to the C1q–/– × C57BL/6 model and was shown to improve BP and vascular function both at GD 17.5 as well as in the postpartum period (31).
Interleukin-10 –/– and Interleukin-4–/– Mouse Models
IL-4 and IL-10 are typically characterized as anti-inflammatory cytokines that are produced by a wide range of both immune and nonimmune cell types. IL-4 is needed for the polarization of naive T-helper cells into Th2 cells. IL-4 increases throughout pregnancy, perhaps due to the increases in progesterone (112), but women with PE reportedly have lower levels of circulating IL-4 and increased levels of the soluble IL-4 receptor (113, 114). IL-10 was initially identified by its ability to inhibit T-cell effector functions, and it is now known to play a role in the differentiation of naive T cells into Treg cells and inhibit antigen presentation by downregulating MHC class II and costimulatory molecule expression. Pups born to mice deficient in IL-4 have normal fetal growth and development (115, 116); however, pregnant IL-4–/– mice have immune system activation, increased inflammation in the placenta as well as HTN and proteinuria (39). Similarly, IL-10–/– mice had normal pregnancies in regard to pups per litter and lack of fetal demise (38, 116), but had a slight increase in BP and isolated aortas had impaired relaxation responses to acetylcholine. In IL-4–/– and IL-10–/– mice, the preeclamptic symptoms were exacerbated when the TLR3 mimetic CpG was given during pregnancy (38, 39).
Tumor Necrosis Factor-α Infusion
TNF-α is commonly cited as a central mediator in inflammation, and elevated levels of TNF are present both in the serum and placental tissue from PE mothers (117). Various animal models have been developed in which pregnant dams are given TNF-α during pregnancy. Alexander et al (32) infused TNF-α in pregnant Sprague-Dawley rats from GD14 to GD19, which led to a significant increase in mean arterial pressure (MAP). TNF infusion did not affect pup weight or litter size. In follow-up studies, renal hemodynamics were assessed, and TNF-α infusion decreased glomerular filtration rate (GFR), renal plasma flow (RPF), and renal plasma resistance (33). Heme oxygenase induction with cobalt protoporphyrin IX chloride lowered BP and sFlt-1 levels in the rat TNF infusion model (118). Endothelin receptor A antagonism lowered BP and TNF-α levels in this model as well (34). In a mouse model, C57BL/6 mice were administered TNF-α via a subcutaneously implanted miniosmotic pump beginning at GD13. TNF-α treatment led to a robust increase in BP and proteinuria. There was also an increase in hypoxia-inducible factor 1α (HIF-1α) expression in the placentas. Unlike in the rat, mice administered TNF-α do not have an increase in circulating sFlt-1 (35). Similar to the low-dose LPS or TLR stimulation models, infusion of the same doses of TNF-α to virgin rats or mice does not elicit the same increases in BP or renal injury. Pregnant baboons develop a PE-like syndrome when infused with TNF-α for 2 weeks during midpregnancy. BP was elevated during the 2 weeks of TNF-α infusion. The baboons had elevated sFlt-1 in the circulation and in the placenta that remained elevated post TNF infusion. Similar to rats and mice, there was no evidence of IUGR in the baboons (36). Of note, an additional inflammatory cytokine, IL-6, has also been successfully used to induce a PE phenotype during pregnancy, while having no effect on virgin rats (119). Treatment with the angiotensin type 1 receptor antagonist, losartan abolished the increases in BP in response to IL-6 in pregnant rats (120).
Spontaneous/Genetic Models
BPH/5 Mouse
A cross-breeding program was undertaken in the 1970s using 8 different inbred mice strains to generate mice with low (BPL/1), normal (BPN/3), and high BP (BPH/2), as assessed by tail-cuff BP measurements (40). The BPH/5 mouse is an subline of the BPH/2 strain that exhibits mild BP elevation as an adult (40), as well as hyperphagia, obesity, and leptin resistance (121). In addition, BPH/5 mice have low circulating estrogen and irregular estrous cycles. When mated in brother-sister fashion, pregnant BPH/5 mice exhibit many of the hallmark characteristics of PE, including HTN and proteinuria in late pregnancy that resolves after delivery of the pups (40). Because of the preexisting HTN in the BPH/5, this can be considered a model of superimposed PE. Examination of placental development in BPH/5 mice showed that abnormalities in the placental unit precede the development of HTN and proteinuria. Specifically, the placentas of BPH/5 mice are significantly smaller throughout midpregnancy and also have abnormal spiral artery remodeling. BPH/5 dams have evidence of placental reactive oxygen species (ROS) at midgestation, and reduced expression and activity of superoxide dismutase in the placenta. Treatment with the ROS scavenger Tempol leads to a reduction in maternal BP as well as an improvement in fetoplacental outcomes (41). In addition, angiogenic imbalance has also been examined in the BPH/5 model. Compared to pregnant C57BL/6 mice, pregnant BPH/5 mice have decreased circulating free VEGF and placental growth factor (PlGF). Adenoviral delivery of VEGF121, the predominant VEGF isoform at embryonic day 7.5, restored plasma VEGF levels, decreased fetal resportions, and reduced BP (122). Before implantation, there is an upregulation of cyclooxygenase-2 (COX-2) and IL-15 at the maternal-fetal interface, which was associated with decreased decidual NK cells. Treatment with the selective Cox2 inhibitor celecoxib improved the NK cell numbers and improved maternal HTN and fetal outcomes (42). A recent genotypic analysis of the BPH/5 mouse revealed that the majority of differences between the genomes of the non-PE BPH/2 mouse and the BPH/5 mouse involved innate and adaptive immunity including complement activation and lymphocyte regulation (123). These genomic data support a previous study in which the BPH/5 mouse was shown to have excess complement activation at the maternal/fetal interface, leading to excess neutrophil accumulation. Treatment with complement inhibitors CR2-Crry, which inhibits all complement pathways, or CR2, which is specific for the alternative pathway, led to improvements in placentation and decreased fetal demise (43).
Dahl Salt-sensitive Rat
The Dahl salt-sensitive (Dahl S) rat is a widely used model of salt-sensitive HTN and chronic kidney disease (124), 2 conditions that greatly increase the risk of developing PE in humans (125, 126). Dahl S rats progressively develop HTN and renal injury while maintained on a normal chow diet; however, this is exacerbated when administered a high-salt diet. Gillis and colleagues (44) characterized pregnancy in the Dahl S, while maintaining the rats on a normal chow (0.3% NaCl) diet. When pregnant, the Dahl S develops exacerbated HTN and a severe increase in urinary protein excretion. Dahl S also have increased uterine artery resistance and evidence of placental hypoxia, including increased placental HIF1-α and TNF-α. The Dahl S pregnancy is also characterized by fetal growth restriction and decreased litter size. When fed a high-salt diet during pregnancy, BP is increased further, and the downstream effects of reduced uteroplacental perfusion are exacerbated (45). Several therapeutics have been tested in the Dahl S model of superimposed PE, including phosphodiesterase 5 (PDE5) inhibition with sildenafil. Sildenafil treatment during pregnancy improved BP, renal injury, and fetal growth (46). It should be noted, however, that human studies using sildenafil have proved disappointing. Results from the STRIDER trial failed to demonstrate a beneficial effect, though it is noted that this was low-dose sildenafil and administered only relatively late in gestation (127). More seriously, questions have arisen as to the safety of sildenafil administration in at least one arm of the trial (128). A very recent study showed that the source of dietary protein given to the Dahl S rat affects the PE phenotype; rats given a casein-based diet develop PE roughly 50% of the time, whereas rats that ate a diet with a wheat gluten protein source did not develop PE (129). Diet and subsequently the gut microbiota could have an important effect on the production of microbial metabolites as well as the balance of tolerance and inflammation in the immune system, which may affect the development and outcome of PE both in animal models and patients. Evidence continues to mount supporting a pathogenic role for the microbiome in chronic inflammatory diseases; thus it may be that diet should be an important consideration in PE patients.
Surgical and Pharmacological Models
N-Nitro-L-Arginine Methyl Ester Infusion
A healthy pregnancy is characterized by volume expansion, vasodilation, and decreased BP. These changes correlate with increases in urinary excretion of NO2 and NO3, reflecting increases in NO production (50). Treatment of rats with the nitric oxide synthase (NOS) inhibitor L-NAME during pregnancy results in HTN, proteinuria, thrombocytopenia, and IUGR, thus clearly mimicking the syndrome of PE (51, 52). The L-NAME can be administered in drinking water (53) or via continuous venous infusion (51). Further, this model has been generated in mice as well (130). Recently, Soobryan and colleagues (54) administered L-NAME on GD4-8 to mimic early-onset PE and on GD8-14 to model late-onset PE. Interestingly, mice that lack endothelial or constitutive NOS (NOS3) do not develop HTN, but do have fetal growth restriction, a reduction in uterine blood flow, and placental hypoxia (131-134). Several therapeutics that increase NO have been used successfully in the L-NAME infusion model, including sildenafil and L-arginine (52, 53, 55).
Arginine Vasopressin Infusion
An interesting new avenue of research opened with the discovery that vasopressin might be dysregulated in PE (135). Ectopic administration of vasopressin in pregnant mice resulted in all of the traditional hallmarks of PE: HTN, proteinuria, and glomerular endotheliosis. This phenotype is perhaps not surprising. What was much more intriguing were follow-up studies indicating unlikely links between vasopressin and other, seemingly unrelated, pathways. One study demonstrated that vasopressin excess resulted in altered placental morphology, placental reactive oxygen, and decreased PlGF in the absence of sFlt-1 changes, thus altering angiogenic balance (60). Perhaps the most intriguing report originating from this model is the substantial effect on T-helper cell populations that is consistent with the changes seen in PE patients. It was further noted that vasopressin receptor 1a was decreased in CD4 + T cells and that circulating proinflammatory cytokines were increased after vasopressin administration in pregnant mice (61). Though not as thoroughly characterized as some other models, the clinical data and follow-up mechanistic research suggest vasopressin dysregulation could be an intriguing future area of PE research.
Soluble fms-like Tyrosine Kinase-1 Infusion/Overexpression
Given the well-characterized alterations in the expression of proangiogenic and antiangiogenic factors in PE, the use of models that rely on altering this balance have a clear rationale. VEGF, besides its role in blood vessel formation, is an important factor in maintaining endothelial health, particularly in the kidney (136). One of the endogenous antagonists of VEGF is a soluble splice variant of the VEGF receptor-1 (sFlt-1), which acts as a VEGF decoy receptor and a dominant-negative receptor of endogenous full-length VEGF receptors (137). The groundbreaking work from Maynard et al (62) found that sFlt-1 was significantly elevated in PE, and it is well established that loss of VEGF signaling in patients undergoing chemotherapeutic anti-VEGF therapy is associated both with HTN and proteinuria (138).
Several direct model systems have demonstrated the effects of sFlt-1 excess in producing a PE-like phenotype. In the original report of sFlt-1 excess in PE from Maynard and colleagues in the Karumanchi research group (62), it was found that viral overexpression of sFlt-1 in pregnant mice resulted in a PE phenotype; including HTN, proteinuria, and renal injury, findings that have been verified and expanded on by other laboratory groups (139). Other approaches have involved infusing chimeric Fc–sFlt-1 proteins directly into pregnant animals (63). Regardless of the origin of the sFlt-1 excess, there are noted increases in oxidative stress (64), endothelial dysfunction (65), and renal injury (66). Several therapies have been tested in the various sFlt-1 models, including pravastatin, which increased PlGF levels and improved placentation and fetal growth restriction (140). Treatment with Tempol to decrease oxidative stress or endothelin receptor type A blockade both improve the symptoms of PE in this model (65, 141).
Although perhaps not as well characterized as sFlt-1, there is a plethora of evidence that soluble endoglin (sEng) plays a major role in PE. sEng, a soluble receptor for transforming growth factor β has been shown to be significantly upregulated in PE and correlate directly with disease severity (142). In an interesting study, sEng was delivered by adenoviral expression in pregnant rats, which resulted in HTN, fetal growth restriction, and increased renal injury. Further, administration of adenoviral-derived sEng and sFlt-1 together led to hemolysis, elevated liver enzymes, and a decrease in platelets, suggesting that this model could be used to simulate HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome (143). This suggests not only that sEng excess could be a viable model for PE, but that the synergistic effects of sFlt-1 and sEng could be of great value in studying the more acutely dangerous HELLP syndrome.
Placental Ischemia
Early work in humans revealed that deficient invasion of the maternal spiral arteries feeding the increasingly hungry fetal/placental unit were commonly found in cases of PE (144). Subsequently, a number of early studies using animal models demonstrated that restriction of blood flow to the placenta could mimic many of the features of PE. The earliest indication came from dog studies in the late 1960s, in which partial occlusion of the utero-ovarian arteries resulted in HTN. Tellingly, the banding occurred before pregnancy, the HTN manifested only during gestation, and resolved post partum (145). Subsequent studies suggested the presence of glomerular endotheliosis, consistent with renal lesions seen in PE patients (146). In similar subsequent studies in pregnant rabbits, besides the expected HTN, there was also a demonstrated increase in peripheral resistance, similar to that seen in PE (147). Taken together, these reports strongly suggested a link between reduced uteroplacental blood flow and gestational HTN and symptoms consistent with PE.
Nor were these observations restricted to lower vertebrates. A series of studies found similar results in nonhuman primates (NHPs). In studies mirroring the earlier dog experiments, ligation of the gonadal arteries with restrictive banding of the uterine arteries resulted in characteristic increases in BP, proteinuria, reduced renal artery, and an increase in markers of renal injury, as evidenced by increased fibrin/fibrinogen deposition (148). More recent studies have extended these findings in baboons, with radiotelemetry allowing for real-time, longitudinal studies of BP throughout pregnancy. In this model, the uterine artery ligation (UAL) of one uterine artery at approximately two-thirds of the way to term, resulted in a roughly 40% reduction in overall uteroplacental blood flow. As a result, maternal BP significantly increased as determined by radiotelemetry, and remained elevated throughout the pregnancy. In addition, the baboons developed increased maternal protein:creatinine ratio, and electron microscopy indicated signs of both endotheliosis and mesangial expansion. Finally, immediately post ligation, there was an immediate increase in the circulating levels of sFlt-1, which remained high throughout the pregnancy, thus highlighting the importance of placental ischemia in regulating sFlt-1 (67).
This model has also been used to test potential therapeutic approaches for targeting sFlt-1 in PE. In the first, recombinant human PlGF was administered subcutaneously for 5 days after 2 weeks of UAL. This treatment resulted in significant decreases both in maternal BP and proteinuria, highlighting the importance of sFlt-1 in mediating the renal injury induced by placental ischemia (68). In a more recent report, Turanov et al (69) used stabilized short interfering RNA (siRNA) to selectively target the 3′ untranslated regions of the 2 most abundant sFlt-1 transcripts (i13 and e15), which are also present in the baboon. As a result, circulating sFlt-1 was reduced by nearly half, and HTN and proteinuria were both completely normalized. These studies demonstrate both the important role of placental ischemia in inducing a PE phenotype and a demonstration of the NHP model to serve as a drug development model.
The baboon UAL model is not without its drawbacks. Experience with primates is relatively rare, very expensive, and slow, because of the time needed to establish breeding and the long gestation. For that reason, the rodent reduced uterine perfusion pressure (RUPP) model of placental ischemia has become one of the most prevalent models in use in preclinical laboratories. First described (70) and extensively characterized by Alexander and colleagues in the Granger laboratory, the RUPP model has demonstrated many similarities to human PE. In short, using Sprague Dawley rats on GD14 of a pregnancy, restrictive silver clips of fixed width are placed on the gonadal arteries caudal to the ovaries, as well as the lower abdominal aorta caudal to the renal arteries, both routes that supply the gravid uterus. This results in an approximately 40% reduction in blood flow to the uterus and placentas (149), a roughly 12% decrease in placental oxygen saturation (150), and an approximately 15 to 20 mm Hg increase in MAP (71). Similar to the PE state, it has been demonstrated that RUPP rodents also demonstrate proteinuria, altered RPF and GFR, and a distinct hypertensive shift in the pressure/natriuresis relationship (70). Perhaps most important, the RUPP rat demonstrates the endothelial dysfunction believed to underlie the maternal syndrome (151), increased peripheral resistance (149), as well as alterations in myogenic tone (152, 153). Other similarities include increased production of inflammatory cytokines like IL-6 (119) and TNF-α (33) seen in preeclamptic patients. Finally, the RUPP model exhibits tell-tale evidence of placental oxidative stress, also consistent with evidence for elevated placental oxidative stress in PE (154). Supporting this, the RUPP rat has increased levels of 8-isoprostane (72) and placental superoxide levels (63). Further, several interventions targeting ROS have demonstrated a beneficial effect on maternal HTN (72-74). Similar to the BPH/5 mouse, administration of the superoxide dismutase mimetic Tempol lowered BP in RUPP rats and also attenuated fetal growth restriction (72). In addition, induction of heme oxygenase 1 using cobalt protoporphyrin also significantly decreased placental superoxide production in RUPP animals (73).
Like the aforementioned NHP models of placental ischemia, the RUPP model has been used to test interventional strategies for therapeutic development. Two independent reports demonstrated that chronic VEGF administration lowered BP, improved renal function, and helped normalize endothelial dysfunction in this model (66, 75). Furthermore, a biopolymer-stabilized recombinant VEGF chimera has been used in the RUPP model. This treatment does not cross the placental barrier at therapeutic doses and was effective at reducing maternal BP and reduced free sFlt-1 in the plasma (76). Interventions targeting inflammation, specific inflammatory cytokines and immune cell subsets, and oxidative stress have also shown beneficial effect (33, 72, 73, 155-157). Regal and colleagues (158) have examined the role of complement activation in the development of HTN in RUPP pregnancy and found that complement and immunoglobulin M deposition was increased in the placenta and kidney of RUPP rats. They also found that complement inhibition lowered BP in RUPP animals (159). Though problematic from a pharmacological standpoint because of potential teratogenicity, it was also demonstrated that blockade of the endothelin-1 receptor A significantly ameliorated the symptoms associated with the RUPP model (71). Recently, our group has used the RUPP model to interrogate the beneficial effects of stabilized chimeric therapeutic peptides targeting both the sFlt-1 protein and the master inflammatory mediator NFκB (76, 160). These studies revealed that targeting both of these pathways with pharmacologically relevant agents could attenuate the maternal symptoms of placental ischemia.
Alternatives to the traditional RUPP model have also been described in the literature. Morton et al of the Davidge research group (161) modified the standard procedure in what they termed the selected RUPP model (sRUPP). This model forgoes the abdominal clip in favor of placing individual clips on the uterine arteries near the site they branch from the internal iliac artery, in an effort to prevent hindlimb ischemia. Very similar to the RUPP model, the sRUPP exhibits increased MAP, oxidative stress, inflammation, and increased endoglin, although interestingly no changes in either sFlt-1 or VEGF were noted. There have also been several reports using murine models of placental ischemia, with varying methodology. Intapad et al (162) used similar methodology as the rodent RUPP model, partially restricting blood flow through both the uterine arteries and the abdominal aorta. This resulted in not only HTN, but like the rat RUPP, increases in circulating sFlt-1 and fetal growth restriction. Fushima and colleagues (163) took an alternative approach, performing bilateral ligation of the ovarian arteries, the uterine arteries, or both on GD14.5. This resulted in a severe decrease in uterine blood flow and marked HTN.
This model was later used to demonstrate a beneficial effect of nicotinamide on placental ischemia-induced HTN, proteinuria, and renal injury (164).
Conclusions
PE remains one of the most vexing obstetric disorders, and includes mechanistic aspects that involve vastly different pathways. What is clear from the literature is there is dysfunction arising from the angiogenic imbalance, immune activation, and endothelial dysfunction. A difficulty in studying these pathways in pregnancy in humans is both real and perceived risk to the mother and offspring. Isolation of these pathways in animal models allows us to deconvolute the relative importance of each specific pathway. Meanwhile, the use of models of placental ischemia allows for a more holistic view of placental dysfunction during PE. Moving forward, these animal models will be crucial tools for the discovery of effective therapeutics for the management of PE patients and the health of the baby. Already, interventions that have ranged from dietary to pharmacological have been attempted in many of these models and have yielded promising results, some of which have begun to be translated to the patient population. The use of animal models for PE has yielded many new insights and will provide an important resource for the further understanding of the complex etiology that underlies the disease.
Glossary
Abbreviations
- BP
blood pressure
- COX-2
cyclooxygenase-2
- Dahl S
Dahl salt-sensitive
- DAMP
danger-associated molecular pattern
- ET-1
endothelin-1
- GD
gestational day
- GFR
glomerular filtration rate
- HELLP syndrome
hemolysis, elevated liver enzymes, and low platelets
- HIF-1α
hypoxia-inducible factor 1α
- IL
interleukin
- IUGR
intrauterine growth restriction
- L-NAME
N-nitro-L-arginine methyl ester
- LPS
lipopolysaccharide
- MAP
mean arterial pressure
- MHC
major histocompatibility complex
- NF
nuclear factor
- NHP
nonhuman primate
- NK
natural killer
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE5
phosphodiesterase 5
- PE
preeclampsia
- PlGF
placental growth factor
- ROS
reactive oxygen species
- RPF
renal plasma flow
- RUPP
reduced uterine perfusion pressure
- sEng
soluble endoglin
- sFlt-1
soluble fms-like tyrosine kinase-1
- siRNA
small interfering RNA
- sRUPP
selected RUPP
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- Treg
regulatory T cells
- UAL
uterine artery ligation
- VEGF
vascular endothelial growth factor
Contributor Information
Erin B Taylor, Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi 39216-4505, USA.
Eric M George, Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi 39216-4505, USA.
Financial Support
This work was supported by the National Institutes of Health- National Heart Lung and Blood Institute (NIH; grant Nos. NHLBI R00HL146888 to E.B.T., NIH NHLBI R01HL137791 to E.M.G., and National Institutes of Health- National Institute of General Medical Sciences P20GM104357 to the UMMC Department of Physiology and Biophysics).
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122-1131. [DOI] [PubMed] [Google Scholar]
- 2. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094-1112. [DOI] [PubMed] [Google Scholar]
- 3. Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am J Physiol Renal Physiol. 2020;318(6):F1315-F1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts JM, Rich-Edwards JW, McElrath TF, Garmire L, Myatt L; Global Pregnancy Collaboration. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021;77(5):1430-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiong X, Fraser WD. Impact of pregnancy-induced hypertension on birthweight by gestational age. Paediatr Perinat Epidemiol. 2004;18(3):186-1 91. [DOI] [PubMed] [Google Scholar]
- 6. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 7. Guedes-Martins L. Superimposed preeclampsia. Adv Exp Med Biol. 2017;956:409-417. [DOI] [PubMed] [Google Scholar]
- 8. Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9(3):147-60. [DOI] [PubMed] [Google Scholar]
- 9. Bartsch E, Medcalf KE, Park AL, Ray JG; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dekker GA. Management of preeclampsia. Pregnancy Hypertens. 2014;4(3):246-247. [DOI] [PubMed] [Google Scholar]
- 11. Faas MM, Schuiling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol. 1994;171(1):158-164. [DOI] [PubMed] [Google Scholar]
- 12. Faas MM, Schuiling GA, Baller JF, Bakker WW. Glomerular inflammation in pregnant rats after infusion of low dose endotoxin. An immunohistological study in experimental pre-eclampsia. Am J Pathol. 1995;147(5):1510-1518. [PMC free article] [PubMed] [Google Scholar]
- 13. Faas MM, Broekema M, Moes H, van der Schaaf G, Heineman MJ, de Vos P. Altered monocyte function in experimental preeclampsia in the rat. Am J Obstet Gynecol. 2004;191(4):1192-1198. [DOI] [PubMed] [Google Scholar]
- 14. Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xue P, Zheng M, Gong P, et al. . Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PLoS One. 2015;10(4):e0124001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gong P, Liu M, Hong G, et al. . Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta. 2016;41:45-52. [DOI] [PubMed] [Google Scholar]
- 17. Lin F, Zeng P, Xu Z, et al. . Treatment of Lipoxin A(4) and its analogue on low-dose endotoxin induced preeclampsia in rat and possible mechanisms. Reprod Toxicol. 2012;34(4):677-685. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Yang J, Bao J, et al. . Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta. 2017;49:23-32. [DOI] [PubMed] [Google Scholar]
- 19. Li G, Ma L, Lin L, et al. . The intervention effect of aspirin on a lipopolysaccharide-induced preeclampsia-like mouse model by inhibiting the nuclear factor-kappaB pathway. Biol Reprod. 2018;99(2):422-432. [DOI] [PubMed] [Google Scholar]
- 20. Hu J, Zhang J, Zhu B. Protective effect of metformin on a rat model of lipopolysaccharide-induced preeclampsia. Fundam Clin Pharmacol. 2019;33(6):649-658. [DOI] [PubMed] [Google Scholar]
- 21. Li Q, Yin L, Si Y, et al. . The bioflavonoid quercetin improves pathophysiology in a rat model of preeclampsia. Biomed Pharmacother. 2020;127:110122. [DOI] [PubMed] [Google Scholar]
- 22. Li G, Wei W, Suo L, et al. . Low-dose aspirin prevents kidney damage in LPS-induced preeclampsia by inhibiting the WNT5A and NF-kappaB signaling pathways. Front Endocrinol (Lausanne). 2021;12:639592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatterjee P, Weaver LE, Doersch KM, et al. . Placental toll-like receptor 3 and toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. 2012;7(7):e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009;22(12):1314-1319. [DOI] [PubMed] [Google Scholar]
- 25. Chatterjee P, Chiasson VL, Seerangan G, et al. . Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. 2015;28(1):135-142. [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee P, Chiasson VL, Seerangan G, et al. . Depletion of MHC class II invariant chain peptide or gamma-delta T-cells ameliorates experimental preeclampsia. Clin Sci (Lond). 2017;131(15):2047-2058. [DOI] [PubMed] [Google Scholar]
- 27. Goulopoulou S, Wenceslau CF, McCarthy CG, Matsumoto T, Webb RC. Exposure to stimulatory CpG oligonucleotides during gestation induces maternal hypertension and excess vasoconstriction in pregnant rats. Am J Physiol Heart Circ Physiol. 2016;310(8):H1015-H 1025. [DOI] [PubMed] [Google Scholar]
- 28. Osikoya O, Jaini PA, Nguyen A, Valdes M, Goulopoulou S. Effects of low-dose aspirin on maternal blood pressure and vascular function in an experimental model of gestational hypertension. Pharmacol Res. 2017;120:267-278. [DOI] [PubMed] [Google Scholar]
- 29. Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;58(4):716-724. [DOI] [PubMed] [Google Scholar]
- 30. Sutton EF, Gemmel M, Brands J, Gallaher MJ, Powers RW. Paternal deficiency of complement component C1q leads to a preeclampsia-like pregnancy in wild-type female mice and vascular adaptations postpartum. Am J Physiol Regul Integr Comp Physiol. 2020;318(6):R1047-R1057. [DOI] [PubMed] [Google Scholar]
- 31. Gemmel M, Sutton EF, Brands J, Burnette L, Gallaher MJ, Powers RW. l-Citrulline supplementation during pregnancy improves perinatal and postpartum maternal vascular function in a mouse model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2021;321(3):R364-R376. [DOI] [PubMed] [Google Scholar]
- 32. Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15(2 Pt 1):170-175. [DOI] [PubMed] [Google Scholar]
- 33. LaMarca BBD, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46(4):1022-1025. [DOI] [PubMed] [Google Scholar]
- 34. LaMarca BBD, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82-86. [DOI] [PubMed] [Google Scholar]
- 35. Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental regulation of inflammation and hypoxia after TNF-α infusion in mice. Am J Reprod Immunol. 2015;74(5):407-418. [DOI] [PubMed] [Google Scholar]
- 36. Sunderland NS, Thomson SE, Heffernan SJ, et al. . Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine. 2011;56(2):192-199. [DOI] [PubMed] [Google Scholar]
- 37. LaMarca BB, Cockrell K, Sullivan E, et al. . Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82-86. [DOI] [PubMed] [Google Scholar]
- 38. Chatterjee P, Chiasson VL, Kopriva SE, et al. . Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. 2011;58(3):489-496. [DOI] [PubMed] [Google Scholar]
- 39. Chatterjee P, Kopriva SE, Chiasson VL, et al. . Interleukin-4 deficiency induces mild preeclampsia in mice. J Hypertens. 2013;31(7):1414-1423. [DOI] [PubMed] [Google Scholar]
- 40. Davisson RL, Hoffmann DS, Butz GM, et al. . Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39(2 Pt 2):337-342. [DOI] [PubMed] [Google Scholar]
- 41. Hoffmann DS, Weydert CJ, Lazartigues E, et al. . Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension. 2008;51(4):1058-1065. [DOI] [PubMed] [Google Scholar]
- 42. Sones JL, Cha J, Woods AK, et al. . Decidual Cox2 inhibition improves fetal and maternal outcomes in a preeclampsia-like mouse model. JCI Insight. 2016;1(3):e75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gelber SE, Brent E, Redecha P, et al. . Prevention of defective placentation and pregnancy loss by blocking innate immune pathways in a syngeneic model of placental insufficiency. J Immunol. 2015;195(3):1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309(1):R62-R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takushima S, Nishi Y, Nonoshita A, et al. . Changes in the nitric oxide-soluble guanylate cyclase system and natriuretic peptide receptor system in placentas of pregnant Dahl salt-sensitive rats. J Obstet Gynaecol Res. 2015;41(4):540-550. [DOI] [PubMed] [Google Scholar]
- 46. Gillis EE, Mooney JN, Garrett MR, Granger JP, Sasser JM. Sildenafil treatment ameliorates the maternal syndrome of preeclampsia and rescues fetal growth in the Dahl salt-sensitive rat. Hypertension. 2016;67(3):647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Terstappen F, Clarke SM, Joles JA, et al. . Sodium thiosulfate in the pregnant dahl salt-sensitive rat, a model of preeclampsia. Biomolecules. 2020;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Man AWC, Zhou Y, Lam UDP, et al. . l-Citrulline ameliorates pathophysiology in a rat model of superimposed preeclampsia. Br J Pharmacol. 2021 [DOI] [PubMed]
- 49. Periconceptional 1,3-butanediol supplementation suppresses the superimposed preeclampsia-like phenotype in the Dahl salt-sensitive rat. Am J Physiol Heart Circ Physiol. 2022;322(2):H285-H295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deng A, Engels K, Baylis C. Impact of nitric oxide deficiency on blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int. 1996;50(4):1132-1138. [DOI] [PubMed] [Google Scholar]
- 51. Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol. 1994;170(5 Pt 1):1458-1466. [DOI] [PubMed] [Google Scholar]
- 52. Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of L-arginine, D-arginine and steroid hormones. Hum Reprod. 1995;10(10):2723-2730. [DOI] [PubMed] [Google Scholar]
- 53. Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate improves fetal outcomes in pregnant, L-NAME treated, Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):22-26. [DOI] [PubMed] [Google Scholar]
- 54. Soobryan N, Murugesan S, Phoswa W, Gathiram P, Moodley J, Mackraj I. The effects of sildenafil citrate on uterine angiogenic status and serum inflammatory markers in an L-NAME rat model of pre-eclampsia. Eur J Pharmacol. 2017;795:101-107. [DOI] [PubMed] [Google Scholar]
- 55. Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):136-140. [DOI] [PubMed] [Google Scholar]
- 56. Motta C, Grosso C, Zanuzzi C, et al. . Effect of sildenafil on pre-eclampsia-like mouse model induced by L-name. Reprod Domest Anim. 2015;50(4):611-616. [DOI] [PubMed] [Google Scholar]
- 57. Zheng L, Tang R, Shi L, et al. . Vagus nerve stimulation ameliorates L-NAME-induced preeclampsia-like symptoms in rats through inhibition of the inflammatory response. BMC Pregnancy Childbirth. 2021;21(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martinez-Fierro ML, Hernadez-Delgadillo GP, Flores-Mendoza JF, et al. . Fibroblast growth factor type 2 (FGF2) administration attenuated the clinical manifestations of preeclampsia in a murine model induced by L-NAME. Front Pharmacol. 2021;12:663044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen D, Wang H, Huang H, et al. . Vascular endothelial growth factor attenuates Nomega-nitro-L-arginine methyl ester-induced preeclampsia-like manifestations in rats. Clin Exp Hypertens. 2008;30(7):606-615. [DOI] [PubMed] [Google Scholar]
- 60. Sandgren JA, Deng G, Linggonegoro DW, et al. . Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight. 2018;3(19):e99403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scroggins SM, Santillan DA, Lund JM, et al. . Elevated vasopressin in pregnant mice induces T-helper subset alterations consistent with human preeclampsia. Clin Sci. 2018;132(3):419-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maynard SE, Min JY, Merchan J, et al. . Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. George EM, Arany M, Cockrell K, Storm MV, Stec DE, Granger JP. Induction of heme oxygenase-1 attenuates sFlt-1-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1495-R1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bridges JP, Gilbert JS, Colson D, et al. . Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22(5):564-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Murphy SR, LaMarca BBD, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55(2):394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li Z, Zhang Y, Ying Ma, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50(4):686-692. [DOI] [PubMed] [Google Scholar]
- 67. Makris A, Thornton C, Thompson J, et al. . Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71(10):977-984. [DOI] [PubMed] [Google Scholar]
- 68. Makris A, Yeung KR, Lim SM, et al. . Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension. 2016;67(6):1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Turanov AA, Lo A, Hassler MR, et al. . RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat Biotechnol. 2018;36:1164-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alexander BT, Kassab SE, Miller MT, et al. . Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191-1195. [DOI] [PubMed] [Google Scholar]
- 71. Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37(2 Part 2):485-489. [DOI] [PubMed] [Google Scholar]
- 72. Sedeek M, Gilbert JS, LaMarca BB, et al. . Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21(10):1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57(5):941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Morillon AC, Williamson RD, Baker PN, et al. . Effect of L-ergothioneine on the metabolic plasma profile of the RUPP rat model of pre-eclampsia. PloS One. 2020;15(3):e0230977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension. 2010;55(2):380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Logue OC, Mahdi F, Chapman H, George EM, Bidwell GL III. A maternally sequestered, biopolymer-stabilized vascular endothelial growth factor (VEGF) chimera for treatment of preeclampsia. J Am Heart Assoc. 2017;6(12):e007216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Alexander BT, Llinas MT, Kruckeberg WC, et al. . L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43(4):832-836. [DOI] [PubMed] [Google Scholar]
- 78. LaMarca B, Speed J, Fournier L, et al. . Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52(6):1161-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Spradley FT, Tan AY, Joo WS, et al. . Placental growth factor administration Abolishes placental ischemia-induced hypertension. Hypertension. 2016;67(4):740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Laule CF, Wing CR, Odean EJ, et al. . Effect of nicotine on placental ischemia-induced complement activation and hypertension in the rat. J Immunotoxicol. 2017;14(1):235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Regal JF, Lillegard KE, Bauer AJ, et al. . Neutrophil depletion attenuates placental ischemia-induced hypertension in therRat. PLoS One. 2015;10(7):e0132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7: 320–338. [Google Scholar]
- 83. Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ichijo M. Expression of activation antigens CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology. 1992;75(4):710-712. [PMC free article] [PubMed] [Google Scholar]
- 84. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266-271. [DOI] [PubMed] [Google Scholar]
- 85. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107(20):9299-9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220-223. [DOI] [PubMed] [Google Scholar]
- 88. McMaster MT, Librach CL, Zhou Y, et al. . Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154(8):3771-3778. [PubMed] [Google Scholar]
- 89. King A, Burrows TD, Hiby SE, et al. . Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21(4):376-387. [DOI] [PubMed] [Google Scholar]
- 90. King A, Allan DS, Bowen M, et al. . HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30(6):1623-1631. [DOI] [PubMed] [Google Scholar]
- 91. Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2006;13(5):680-686. [DOI] [PubMed] [Google Scholar]
- 92. Bonney EA. Preeclampsia: a view through the danger model. J Reprod Immunol. 2007;76(1-2):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Minassian C, Thomas SL, Williams DJ, Campbell O, Smeeth L. Acute maternal infection and risk of pre-eclampsia: a population-based case-control study. PLoS One. 2013;8(9):e73047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101(2):227-231. [DOI] [PubMed] [Google Scholar]
- 95. Papageorghiou AT, Deruelle P, Gunier RB, et al. . Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225(3):289.e1-289.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Trogstad LI, Eskild A, Bruu AL, Jeansson S, Jenum PA. Is preeclampsia an infectious disease? Acta Obstet Gynecol Scand. 2001;80(11):1036-1038. [DOI] [PubMed] [Google Scholar]
- 97. Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2008;198(1):7-22. [DOI] [PubMed] [Google Scholar]
- 98. Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1-9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;80(2):243-248. [DOI] [PubMed] [Google Scholar]
- 100. Todt JC, Yang Y, Lei J, et al. . Effects of tumor necrosis factor-alpha on human trophoblast cell adhesion and motility. Am J Reprod Immunol. 1996;36(2):65-71. [DOI] [PubMed] [Google Scholar]
- 101. Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod. 2005;73(2):237-243. [DOI] [PubMed] [Google Scholar]
- 102. Fonseca BM, Correia-da-Silva G, Teixeira NA. The rat as an animal model for fetoplacental development: a reappraisal of the post-implantation period. Reprod Biol. 2012;12(2):97-118. [DOI] [PubMed] [Google Scholar]
- 103. Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for toll-like receptor 3. J Biol Chem. 2004;279(13):12542-12550. [DOI] [PubMed] [Google Scholar]
- 104. Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64(1):27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kang JY, Lee JO. Structural biology of the toll-like receptor family. Annu Rev Biochem. 2011;80:917-941. [DOI] [PubMed] [Google Scholar]
- 106. He B, Yang X, Li Y, et al. . TLR9 (toll-like receptor 9) agonist suppresses angiogenesis by differentially regulating VEGFA (vascular endothelial growth factor A) and sFLT1 (soluble vascular endothelial growth factor receptor 1) in preeclampsia. Hypertension. 2018;71(4):671-680. [DOI] [PubMed] [Google Scholar]
- 107. Girardi G, Lingo JJ, Fleming SD, Regal JF. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front Immunol. 2020;11:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lokki AI, Kaartokallio T, Holmberg V, et al. . Analysis of complement C3 gene reveals susceptibility to severe preeclampsia. Front Immunol. 2017;8:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Agostinis C, Bulla R, Tripodo C, et al. . An alternative role of C1q in cell migration and tissue remodeling: contribution to trophoblast invasion and placental development. J Immunol. 2010;185(7):4420-4429. [DOI] [PubMed] [Google Scholar]
- 110. Christians JK, Leavey K, Cox BJ. Associations between imprinted gene expression in the placenta, human fetal growth and preeclampsia. Biol Lett. 2017;13(11):20170643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Brennan L, Morton JS, Quon A, Davidge ST. Postpartum vascular dysfunction in the reduced uteroplacental perfusion model of preeclampsia. PLoS One. 2016;11(9):e0162487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Marzi M, Vigano A, Trabattoni D, et al. . Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106(1):127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117(3):550-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jonsson Y, Rubèr M, Matthiesen L, et al. . Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70(1-2):83-91. [DOI] [PubMed] [Google Scholar]
- 115. Bonney EA. Maternal tolerance is not critically dependent on interleukin-4. Immunology. 2001;103(3):382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Svensson L, Arvola M, Sällström MA, Holmdahl R, Mattsson R. The Th2 cytokines IL-4 and IL-10 are not crucial for the completion of allogeneic pregnancy in mice. J Reprod Immunol. 2001;51(1):3-7. [DOI] [PubMed] [Google Scholar]
- 117. Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58(1):21-30. [DOI] [PubMed] [Google Scholar]
- 118. George EM, Stout JM, Stec DE, Granger JP. Heme oxygenase induction attenuates TNF-alpha-induced hypertension in pregnant rodents. Front Pharmacol. 2015;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48(4):711-716. [DOI] [PubMed] [Google Scholar]
- 120. LaMarca B, Speed J, Ray LF, et al. . Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Int J Interferon Cytokine Mediat Res. 2011;2011(3):65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sutton EF, Lob HE, Song J, et al. . Adverse metabolic phenotype of female offspring exposed to preeclampsia in utero: a characterization of the BPH/5 mouse in postnatal life. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R485-R491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Woods AK, Hoffmann DS, Weydert CJ, et al. . Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57(1):94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sones JL, Yarborough CC, O’Besso V, Lemenze A, Douglas NC. Genotypic analysis of the female BPH/5 mouse, a model of superimposed preeclampsia. PLoS One. 2021;16(7):e0253453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4(6):753-763. [DOI] [PubMed] [Google Scholar]
- 125. Sibai BM, Lindheimer M, Hauth J, et al. . Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;339(10):667-671. [DOI] [PubMed] [Google Scholar]
- 126. Maruotti GM, Sarno L, Napolitano R, et al. . Preeclampsia in women with chronic kidney disease. J Matern Fetal Neonatal Med. 2012;25(8):1367-1369. [DOI] [PubMed] [Google Scholar]
- 127. Sharp A, Cornforth C, Jackson R, et al. . Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health. 2018;2(2):93-102. [DOI] [PubMed] [Google Scholar]
- 128. Sharp A, Cornforth C, Jackson R, et al. . Mortality in the UK STRIDER trial of sildenafil therapy for the treatment of severe early-onset fetal growth restriction. Lancet Child Adolesc Health. 2019;3(3):e2-e3. [DOI] [PubMed] [Google Scholar]
- 129. Dasinger JH, Abais-Battad JM, Bukowy JD, et al. . Dietary protein source contributes to the risk of developing maternal syndrome in the Dahl salt-sensitive rat. Pregnancy Hypertens. 2021;24:126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ma RQ, Sun MN, Yang Z. Effects of preeclampsia-like symptoms at early gestational stage on feto-placental outcomes in a mouse model. Chin Med J (Engl). 2010;123(6):707-712. [PubMed] [Google Scholar]
- 131. Kulandavelu S, Qu D, Adamson SL. Cardiovascular function in mice during normal pregnancy and in the absence of endothelial NO synthase. Hypertension. 2006;47(6):1175-1182. [DOI] [PubMed] [Google Scholar]
- 132. Shesely EG, Gilbert C, Granderson G, Carretero CD, Carretero OA, Beierwaltes WH. Nitric oxide synthase gene knockout mice do not become hypertensive during pregnancy. Am J Obstet Gynecol. 2001;185(5):1198-1203. [DOI] [PubMed] [Google Scholar]
- 133. Luo K, Thaete LG, Neerhof MG. Endothelin receptor A antagonism and fetal growth in endothelial nitric oxide synthase gene knockout maternal and fetal mice. Reprod Sci. 2016;23(8):1028-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kulandavelu S, Whiteley KJ, Qu D, Mu J, Bainbridge SA, Adamson SL. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension. 2012;60(1):231-238. [DOI] [PubMed] [Google Scholar]
- 135. Santillan MK, Santillan DA, Scroggins SM, et al. . Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64(4):852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ballermann BJ. Contribution of the endothelium to the glomerular permselectivity barrier in health and disease. Nephron Physiol. 2007;106(2):19-25. [DOI] [PubMed] [Google Scholar]
- 137. Wu FTH, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14(3):528-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186-193. [DOI] [PubMed] [Google Scholar]
- 139. Lu F, Longo M, Tamayo E, et al. . The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196(4):396.e1-397.e7. [DOI] [PubMed] [Google Scholar]
- 140. Kumasawa K, Ikawa M, Kidoya H, et al. . Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011;108(4):1451-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Tam KB, LaMarca B, Arany M, et al. . Role of reactive oxygen species during hypertension in response to chronic antiangiogenic factor (sFlt-1) excess in pregnant rats. Am J Hypertens. 2011;24(1):110-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Levine RJ, Lam C, Qian C, et al. . Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992-1005. [DOI] [PubMed] [Google Scholar]
- 143. Venkatesha S, Toporsian M, Lam C, et al. . Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642-649. [DOI] [PubMed] [Google Scholar]
- 144. Khong Y, Brosens I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):301-311. [DOI] [PubMed] [Google Scholar]
- 145. Hodari AA. Chronic uterine ischemia and reversible experimental “toxemia of pregnancy”. Am J Obstet Gynecol. 1967;97(5):597-607. [DOI] [PubMed] [Google Scholar]
- 146. Abitbol MM, Pirani CL, Ober WB, Driscoll SG, Cohen MW. Production of experimental toxemia in the pregnant dog. Obstet Gynecol. 1976;48(5):537-548. [PubMed] [Google Scholar]
- 147. Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci. 1992;303(4):233-240. [DOI] [PubMed] [Google Scholar]
- 148. Cavanagh D, Rao PS, Tung KS, Gaston L. Eclamptogenic toxemia: the development of an experimental model in the subhuman primate. Am J Obstet Gynecol. 1974;120(2):183-196. [DOI] [PubMed] [Google Scholar]
- 149. Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007;293(4):H2080-H2084. [DOI] [PubMed] [Google Scholar]
- 150. Lawrence DJ, Escott ME, Myers L, Intapad S, Lindsey SH, Bayer CL. Spectral photoacoustic imaging to estimate in vivo placental oxygenation during preeclampsia. Sci Rep. 2019;9(1):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35(1 Pt 2):367-372. [DOI] [PubMed] [Google Scholar]
- 152. Reho JJ, Toot JD, Peck J, Novak J, Yun YH, Ramirez RJ. Increased myogenic reactivity of uterine arteries from pregnant rats with reduced uterine perfusion pressure. Pregnancy Hypertens. 2012;2(2):106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ryan MJ, Gilbert EL, Glover PH, et al. . Placental ischemia impairs middle cerebral artery myogenic responses in the pregnant rat. Hypertension. 2011;58(6):1126-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Walsh SW, Vaughan JE, Wang Y, Roberts LJ II. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14(10):1289-1296. [DOI] [PubMed] [Google Scholar]
- 155. Bauer AJ, Banek CT, Needham K, et al. . Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension. 2013;61(5):1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cornelius DC, Hogg JP, Scott J, et al. . Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62(6): 1068-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cornelius DC, Amaral LM, Harmon A, et al. . An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309(8):R884-R891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Regal JF, Strehlke ME, Peterson JM, et al. . Role of IgM and angiotensin II type I receptor autoantibodies in local complement activation in placental ischemia-induced hypertension in the rat. Mol Immunol. 2016;78:38-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lillegard KE, Johnson AC, Lojovich SJ, et al. . Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol. 2013;56(1-2):91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Eddy AC, Howell JA, Chapman H, et al. . Biopolymer-delivered, maternally sequestered NF-κB (nuclear factor-κB) inhibitory peptide for treatment of preeclampsia. Hypertension. 2020;75(1):193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Morton JS, Levasseur J, Ganguly E, et al. . Characterisation of the selective reduced uteroplacental perfusion (sRUPP) model of preeclampsia. Sci Rep. 2019;9(1):9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Intapad S, Warrington JP, Spradley FT, et al. . Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1353-R1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fushima T, Sekimoto A, Minato T, et al. . Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PLoS One. 2016;11(5):e0155426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Fushima T, Sekimoto A, Oe Y, et al. . Nicotinamide ameliorates a preeclampsia-like condition in mice with reduced uterine perfusion pressure. Am J Physiol Renal Physiol. 2017;312(2): F366-F372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.