ABSTRACT
Background: Difficulties in emotion regulation are a core symptom of borderline personality disorder (BPD) and often interfere with cognitive functions, such as working memory (WM). Traumatic childhood experiences, including severe maltreatment, can contribute to emotion dysregulation, possibly mediated by changes in high-frequency heart rate variability (HF-HRV). However, it is not yet entirely understood if HF-HRV alterations underlie impaired WM during emotional distraction in BPD and if this is related to traumatic childhood experiences and to comorbid post-traumatic stress disorder (PTSD).
Objective: Our aim was to investigate performance (reaction times, RTs) and HF-HRV during an emotional working memory task (EWMT) in relation to childhood maltreatment severity and comorbid PTSD in BPD.
Method: Eighty-one women (n = 28 healthy controls (HC) and n = 53 BPD patients of which n = 18 had comorbid PTSD) performed an adapted Sternberg item recognition WM task with neutral and negative social cues (interpersonal scenes from the International Affective Picture System (IAPS), and neutral, fearful, and angry faces) as distractors. Dependent variables were RTs of correct trials and HF-HRV. Childhood maltreatment was assessed with the Childhood Trauma Questionnaire.
Results: Compared to healthy participants, patients with BPD showed prolonged RTs across all distractor conditions with social cues, regardless of their emotional valence. Patients with BPD, especially those with PTSD, demonstrated reduced HF-HRV both at rest and during EWMT. Severity of childhood maltreatment predicted longer RTs and lower HF-HRV during the EWMT.
Conclusions: Findings suggest that adverse childhood experiences accelerate difficulties in shifting attention away from social information and that these are more pronounced in individuals with BPD. Reduced HF-HRV (low parasympathetic-tonus) may be an important psychophysiological mechanism underlying impaired WM in the presence of distracting social cues in patients with BPD, especially in those with comorbid PTSD.
HIGHLIGHTS
This study provides evidence that childhood maltreatment experiences are associated with hypersensitivity to social information and reduced high-frequency heart rate variability during a working memory task in borderline personality disorder.
KEYWORDS: Borderline personality disorder, childhood maltreatment, emotion regulation, emotional working memory, heart rate variability, trauma, working memory
Abstract
Antecedentes: las dificultades en la regulación emocional es un síntoma central del trastorno límite de la personalidad (TLP) y, a menudo, interfieren con las funciones cognitivas, como la memoria de trabajo (MT). Las experiencias traumáticas de la infancia, incluido el maltrato grave, pueden contribuir a la desregulación emocional, posiblemente mediada por cambios en la variabilidad de la frecuencia cardíaca de alta frecuencia (VFC-AF). Sin embargo, aún no se comprende del todo si las alteraciones de VFC-AF subyacen a la alteración de la MT durante la distracción emocional en el TLP y si esto está relacionado con experiencias traumáticas de la infancia y con el trastorno de estrés postraumático (TEPT) comórbido.
Objetivo: Nuestro objetivo fue investigar el rendimiento (tiempos de reacción, TR) y VFC-AF durante una tarea de memoria de trabajo emocional (MTE) en relación con la gravedad del maltrato infantil y el TEPT comórbido en el TLP.
Método: Ochenta y una mujeres (n=28 controles sanos (CS) y n=53 pacientes con TLP, de las cuales n=18 tenían TEPT comórbido) realizaron una tarea de MT de reconocimiento de elementos de Sternberg adaptada con señales sociales neutras y negativas (escenas interpersonales del Sistema internacional de imágenes afectivas (IAPS por sus siglas en ingles) y rostros neutrales, temerosos y enojados) como distractores. Las variables dependientes fueron TR de ensayos correctos y VFC-AF. El maltrato infantil se evaluó con el Cuestionario de Trauma Infantil.
Resultados: En comparación con las participantes sanas, las pacientes con TLP mostraron TR prolongados en todas las condiciones de distracción con señales sociales, independientemente de su valencia emocional. Los pacientes con TLP, especialmente aquellos con TEPT, demostraron una reducción de VFC-AF tanto en reposo como durante MTE. La gravedad del maltrato infantil predijo TR más largos y VFC-AF más bajo durante el MTE.
Conclusiones: Los resultados sugieren que las experiencias infantiles adversas refuerzan las dificultades para desviar la atención de la información social y que estas son más pronunciadas en las personas con TLP. La VFC-AF reducida (tono parasimpático bajo) puede ser un mecanismo psicofisiológico importante subyacente a la MT alterada en presencia de señales sociales que distraen en pacientes con TLP, especialmente en aquellos con TEPT comórbido.
PALABRAS CLAVE: Trastorno Límite de la Personalidad, Maltrato Infantil, Regulación Emocional, Memoria Emocional de Trabajo, Variabilidad del Ritmo Cardíaco, Trauma, Memoria de Trabajo
Abstract
背景: 情绪调节困难是边缘型人格障碍 (BPD) 的一个核心症状,并且经常干扰认知功能,例如工作记忆 (WM)。包括严重虐待在内的创伤性童年经历可能导致情绪失调,这可能由高频心率变异性 (HF-HRV) 改变所中介。然而,目前尚不完全清楚 HF-HRV 改变是否是 BPD 情绪分散期间 WM 受损的基础,以及这是否与创伤性童年经历和并发的创伤后应激障碍 (PTSD) 有关。
目的: 我们旨在考查情绪工作记忆任务 (EWMT) 期间的表现(反应时间,RTs)和 HF-HRV 与 BPD 中童年期虐待严重程度和并发 PTSD 的关系。
方法: 81 名女性(28名 健康对照 (HC) 和 53名 BPD 患者,其中18名 患有并发 PTSD)执行了一项有中性和消极社交线索(来自国际情感图片系统 (IAPS),以及中性、恐惧和愤怒的面孔)作为干扰因素的改编版斯特恩伯格项目识别 WM 任务。因变量是正确试验的 RT 和 HF-HRV。使用童年期创伤问卷评估童年期虐待。
结果: 与健康参与者相比,BPD 患者在所有带有社交线索干扰条件下都表现出延长的 RT,无论他们的情绪效价如何。 BPD 患者,尤其患有 PTSD 的患者,在静息和 EWMT 期间均表现出 HF-HRV 降低。在 EWMT 期间,童年期虐待的严重程度预测了更长的 RT 和更低的 HF-HRV。
结论: 结果表明,不良童年经历加速了将注意力从社交信息转移的困难,并且这些在 BPD 个体中更为明显。降低的 HF-HRV(低副交感神经激活)可能是一个导致 BPD 患者出现分散社交线索这一受损 WM 的重要心理生理机制,尤其是在并发 PTSD 的患者中。
关键词: 边缘性人格障碍, 童年期虐待, 情绪调节, 情绪工作记忆, 心率变异性, 创伤, 工作记忆
1. Introduction
Borderline personality disorder (BPD) is a severe mental disorder, which affects approximately 1–3% of the general population (Trull, Jahng, Tomko, Wood, & Sher, 2010). It is characterized by marked instability in affect, self-image, interpersonal relationships, and behaviour (American Psychiatric Association, 2013). A complex interplay of stressful life experiences (e.g. interpersonal trauma) with genetic, neurobiological, and psychophysiological vulnerabilities is assumed to underlie the development of the disorder (Bohus et al., 2021).
Traumatic childhood experiences, such as severe abuse and neglect, are an important risk factor for BPD. Individuals with the disorder report significantly higher levels of emotional, physical, and sexual abuse than patients with other psychiatric disorders (see Porter et al., 2020). Previous studies found significant associations of childhood maltreatment experiences with overall severity of BPD symptoms, emotion dysregulation, and impulsivity (Cackowski, Neubauer, & Kleindienst, 2016; Carvalho Fernando et al., 2014; Krause-Utz, Erol, et al., 2019). It is not yet fully understood if these associations are partly due to co-occurring post-traumatic stress disorder (PTSD), which is present in 30% of patients with BPD (Pagura et al., 2010). Trauma-related changes in autonomic nervous system functioning may play an important role in this context.
Early and ongoing (traumatic) stress can have adverse effects on autonomic nervous system functioning and has been associated with lower parasympathetic activity (Meyer et al., 2016; Schneider & Schwerdtfeger, 2020). High-frequency HRV (HF-HRV, in the 0.15–0.40 Hz range) or respiratory sinus arrhythmia (RSA) are established measures to evaluate vagally-mediated heart rate, i.e. parasympathetic activity exerted by the vagal nerve relative to activity of the sympathetic nervous system (Grossman & Taylor, 2007; Koenig, Kemp, Feeling, Thayer, & Kaess, 2016).
Previous research suggests that HF-HRV plays an essential role in the flexible adaptation to stressful environmental demands, including emotion regulation (Appelhans & Luecken, 2006; Grossman & Taylor, 2007; Shaffer & Ginsberg, 2017), e.g. in top-down directed emotional control (Sakaki et al., 2016). Higher HRV in general (i.e. considering measures of both time and frequency domains) was linked to better executive functioning (attention) during stressful tasks (Mccraty & Shaffer, 2015). Results of a meta-analysis (Holzman & Bridgett, 2017) support the idea that HRV is an important psychophysiological marker of affective–cognitive functioning (Hansen, Johnsen, & Thayer, 2003; Laborde, Furley, & Schempp, 2015).
Conversely, reduced HF-HRV has been linked to adverse physical and mental health outcomes, including increased risk for immune dysfunction, inflammation, cardiovascular disease (Kemp & Quintana, 2013), affective and anxiety disorders, including PTSD (Kemp, Quintana, Felmingham, Matthews, & Jelinek, 2012; Schneider & Schwerdtfeger, 2020). With regards to BPD, previous research on HF-HRV revealed inconsistencies, partly due to methodological differences in study design and sample characteristics. During rest, patients with BPD showed reduced HRV (Koenig et al., 2016), while changes during emotion regulation tasks have not consistently been found (Dixon-Gordon, Chapman, Lovasz, & Walters, 2011; Fitzpatrick & Kuo, 2016; Krause-Utz, Walther, Lis, Schmahl, & Bohus, 2019; Kuo, Fitzpatrick, Metcalfe, & McMain, 2016; Metcalfe, Fitzpatrick, & Kuo, 2017; Svaldi, Dorn, Matthies, & Philipsen, 2012). Not all studies in BPD controlled for childhood trauma, comorbid PTSD and medication, which may all influence HF-HRV (Krause-Utz, Walther, et al., 2019; Meyer et al., 2016; O’Regan, Kenny, Cronin, Finucane, & Kearney, 2015). Krause-Utz and colleagues (2019) previously found reduced HF-HRV during an emotion regulation task in BPD patients with comorbid PTSD, whereas BPD patients without this comorbidity did not differ from healthy participants. To our knowledge, no study so far has investigated HF-HRV during an emotional working memory task in BPD.
Emotional working memory (eWM) is the ability to employ WM in emotional contexts (e.g. during emotional distraction), which is essential to cope with emotionally distressing information (Koch, Mars, Toni, & Roelofs, 2018). In this respect, eWM is a crucial component of cognitive emotion regulation. It is needed to shift attention away from disturbing material while focusing on goal-directed tasks (Schweizer et al., 2019). A proposed reason for the close association between eWM and emotion regulation is that both functions rely, to a large extent, on a fronto-parietal brain network (e.g. dorsolateral and medial prefrontal cortex), which exerts down-regulatory control of limbic areas (e.g. amygdala) (Denny & Ochsner, 2014; Koch et al., 2018; Ochsner & Gross, 2008). A computerized eWM training has previously shown beneficial effects on emotion regulation capacities in healthy individuals (Schweizer et al., 2019) and in patients with BPD (Krause-Utz et al., 2020).
Understanding and managing emotion dysregulation in BPD is of high importance as individuals with the disorder show strongly fluctuating and intense emotions, experienced as unpredictable and overwhelming (Santangelo et al., 2014). Interpersonal conflicts, such as perceived rejection and abandonment, are strong triggers for emotional distress in BPD (Ebner-Priemer et al., 2008; Sadikaj, Russell, Moskowitz, & Paris, 2010). Maladaptive attempts to regulate emotional distress include non-suicidal self-injury and suicidal behaviour (Bohus et al., 2021; Gunderson, Herpertz, Skodol, Torgersen, & Zanarini, 2018). Improving the understanding of mechanisms that underlie eWM deficits will help to identify subgroups that might profit from targeted interventions or eWM training.
The aim of this study was to investigate associations between eWM performance (reaction times, RTs), HRV, traumatic childhood experiences, and comorbid PTSD, controlling for medication status in BPD. To investigate these research questions, we made use of an extended version of a previously established emotional working memory task (EWMT) with distracting socio-emotional pictures (emotional and neutral naturalistic interpersonal scenes, facial expressions) presented during a brief WM delay interval (Krause-Utz et al., 2012, 2018, 2020, Krause-Utz, Elzinga, Oei, Paret, et al., 2014, Krause-Utz, Elzinga, Oei, Spinhoven, et al., 2014). While performing this task, patients with BPD previously showed more WM deficits when fearful and neutral facial expressions were presented as distractors. At the same time, they did not differ in WM during distractor-free trials (Krause-Utz, Elzinga, Oei, Spinhoven, et al., 2014). Compared to healthy controls, individuals with BPD also showed impaired WM (longer RTs) when distracted by interpersonal scenes (Krause-Utz et al., 2012). Longer RTs during socio-emotional distraction were associated with amygdala hyper-reactivity (Krause-Utz et al., 2012), altered amygdala-frontal co-activity, and increased connectivity in the salience network (Krause-Utz, Elzinga, Oei, Paret, et al., 2014). The current version of the EWMT included angry faces next to fearful and neutral faces and neutral and negative interpersonal scenes as distractors. Previous research suggests that angry faces may be a particularly strong distractor in patients with BPD (Domes et al., 2008; Kaiser, Jacob, Domes, & Arntz, 2016, 2020; Lazarus, Cheavens, Festa, & Zachary Rosenthal, 2014; Veague & Hooley, 2014), especially in those with co-occurring PTSD (Kaiser et al., 2016, 2020). Traumatic experiences, such as severe childhood abuse and neglect, may sensitize vulnerable persons to social cues signalling threat (Downey & Feldman, 1996; Pollak, Vardi, Putzer Bechner, & Curtin, 2005; Seitz et al., 2021).
Based on previous research, we expected WM impairments (longer RTs) after distraction by social cues irrespective of distractor stimulus (hypothesis 1a), and even more so after angry faces (hypothesis 1b). Moreover, we expected significant associations between severity of childhood maltreatment (abuse and neglect), HF-HRV, and RTs during the EWMT. Specifically, we expected a significant direct effect of childhood maltreatment severity on both outcomes (hypothesis 2) and an indirect effect through HF-HRV on RTs (hypothesis 3). In other words, we assumed that severity of childhood maltreatment would predict HF-HRV, which in turn would predict eWM deficits (prolonged RTs during emotional distractors). We further explored the role of comorbid PTSD in this relationship, controlling for medication status as potential confounder.
2. Material and methods
2.1. Participants
Recruitment took place at the Central Institute of Mental Health (CIMH), Mannheim, Germany. Patients were partly recruited via online posts and partly via the outpatient treatment unit of the Institute of Psychiatric and Psychosomatic Psychotherapy at the CIMH. Interested participants were screened for inclusion criteria and then invited for an extensive diagnostic session. General inclusion criteria were age between 18 and 55 and female gender. Exclusion criteria for BPD patients were lifetime history of bipolar affective disorder or psychotic disorder, an acute life-threatening suicidal crisis, mental deficiency and a severe organic disorder. Exclusion criteria for healthy participants were lifetime history of psychiatric disorder and severe organic disorder. The International Personality Disorder Examination (IPDE) (Loranger, 1994) was used to assess BPD diagnosis according to DSM-IV (American Psychiatric Association, 2013). The Structured Interview for DSM-IV (SCID-I) (Lobbestael, Leurgans, & Arntz, 2011) was conducted to detect or rule out comorbidities. Interviews were done by trained clinical psychologists. Symptom severity of BPD was examined using the Borderline Symptom List (BSL-23) (Bohus et al., 2009). Participants further completed a test on verbal intelligence (Multiple choice vocabulary test, MWT) (Lehrl, Triebig, & Fischer, 1995).
Initially, n = 94 women (n = 66 patients with BPD and n = 28 healthy controls) participated in the study. Six patients dropped out before completing the entire study. Data of n = 5 BPD patients and n = 1 HC needed to be excluded from the final analysis because HF-HRV data were contaminated by artefacts. Data from n = 3 more patients, who did not understand the EWMT correctly, had to be excluded post-hoc. Data in n = 13 patients included missing values on the CTQ. The final sample comprised n = 81 women (n = 53 patients, n = 28 HC). Groups did not differ in age, years of education, and intelligence (see Table 1). Eighteen patients (34%) had comorbid PTSD (BPD + PTSD). These patients did not differ in age, education, intelligence, BPD symptom severity or comorbidities other than PTSD from BPD patients without comorbid PTSD (`BPD`), see Table 2. They did, however, report higher levels of childhood trauma (Table 2). Based on established cut-offs for the CTQ (Bernstein et al., 2003; Glaesmer et al., 2013), all patients reported at least one form of severe abuse or neglect, mostly emotional abuse (n = 34; 87%) and emotional neglect (n = 32; 82%), but also physical neglect (n = 24; 62%), physical abuse (n = 16; 41%), and sexual abuse (n = 15; 38%). Patients with BPD + PTSD reported significantly higher levels for all types of abuse and neglect (all p < .0001). In the healthy control group, n = 4 participants reported severe abuse (14% emotional abuse, 4% sexual abuse, 11% physical abuse), while n = 24 reported no to minimal abuse or neglect.
Table 1.
Demographic and clinical characteristics of patients with borderline personality disorder (BPD) and healthy controls (HC).
| BPD (n = 53) Mean ± SD |
HC (n = 28) Mean ± SD |
Group statistics | |
|---|---|---|---|
| Age (years) | 32.19 ± 9.77 | 29.54 ± 7.31 | F(df) = 1.59, p = .211, = .02 |
|
Education level 9 years 10 years 12–13 years |
n = 24 (45%) n = 22 (42%) n = 7 (13%) |
n = 19 (68%) n = 8 (29%) n = 1 (4%) |
Chi²(df) = 4.31, p = .116 |
| Intelligence (MWT-B) | 29.79 ± 3.76 | 29.85 ± 2.89 | F(df) = .01, p = .939, = .00 |
|
BPD sympom severity (BSL-23 mean) |
1.96 ± .74 | .19 ± .18 | F(df) = 155.24, p < .001, = .67 |
| Childhood maltreatment severity (CTQ sum) | 67.85 ± 18.98 | 32.04 ± 11.39 | F(df) = 76.28, p < .001, = .56 |
|
Comorbidities PTSD Major depression Eating disorder Social phobia |
n = 16 (30%) n = 19 (36%) n = 10 (19%) n = 9 (17%) |
– – – – |
Note: BPD = Borderline Personality Disorder patients, BSL-23 = Borderline Symptom List 23; CTQ = Childhood Trauma Questionnaire; HC = Healthy controls, PTSD = Posttraumatic Stress Disorder.
Table 2.
Demographic and clinical characteristics of patients with borderline personality disorder with comorbid posttraumatic stress disorder (BPD + PTSD), patients without comorbid PTSD (BPD), and healthy controls (HC).
| BPD + PTSD (n = 18) |
BPD (n = 35) |
HC (n = 28) |
Group Statistics |
|
|---|---|---|---|---|
| Age [years] | 33.78 ± 9.25 | 31.37 ± 10.07 | 29.54 ± 7.31 | F(2,78) = 1.25, p = .302 |
|
Years of education 9 years 10 years 12–13 years |
n = 3 (17%) n = 7 (39%) n = 8 (44%) |
n = 4 (11%) n = 14 (40%) n = 17 (49%) |
n = 1 (4%) n = 8 (28%) n = 19 (68%) |
Chi² = 4.16, p = .384 |
| MWT-B (intelligence) | 29.55 ± 3.41 | 29.91 ± 3.91 | 29.85 ± 2.84 | F(2,74) = 0.68, p = .934 |
| BSL-23 mean (BPD symptom severity) | 2.17 ± 0.66 | 1.85 ± 0.76 | 0.19 ± 0.18 |
F(2,74) = 81.59, p < .0001 BPD vs. HC, p < .0001 BPD + PTSD vs. HC, p < .0001 BPD vs. BPD + PTSD, p = .169 |
| CTQ (Childhood trauma) | 77.24 ± 18.37 | 62.72 ± 9.93 | 32.04 ± 11.39 |
F(2,55) = 87.19, p < .0001 BPD vs. HC, p < .0001 BPD + PTSD vs. HC, p < .0001 BPD vs. BPD + PTSD, p < .0001 |
| Comorbidities | ||||
| Major depression lifetime | n = 17 | n = 31 | Chi² = 1.17, p = .280 | |
| Dysthymia lifetime | n = 3 | n = 8 | Chi² = 1.20, p = .274 | |
| Alcohol abuse lifetime | n = 5 | n = 8 | Chi² = 1.41, p = .493 | |
| Substance abuse lifetime | n = 6 | n = 9 | Chi² = 0.22, p = .642 | |
| Dependence lifetime | n = 6 | n = 10 | Chi² = 0.06, p = .812 | |
| Panic disorder current | n = 6 | n = 3 | Chi² = 2.66, p = .103 | |
| Panic disorder lifetime | n = 6 | n = 3 | Chi² = 2.66, p = .103 | |
| Social phobia current | n = 7 | n = 6 | Chi² = 0.73, p = .393 | |
| Social phobia lifetime | n = 7 | n = 6 | Chi² = 0.73, p = .393 | |
| Specific phobia current | n = 3 | n = 2 | Chi² = 0.66, p = .416 | |
| Specific phobia lifetime | n = 3 | n = 2 | Chi² = 0.66, p = .416 | |
| OCD current / lifetime | n = 2 | n = 2 | Chi² = 0.11, p = .735 | |
| Pain disorder lifetime | n = 0 | n = 1 | Chi² = 1.54, p = .464 | |
| Anorexia current | n = 0 | n = 1 | Chi² = 0.70, p = .403 | |
| Anorexia lifetime | n = 5 | n = 6 | Chi² = 0.12, p = .725 | |
| Bulimia current | n = 2 | n = 8 | Chi² = 2.22, p = .136 | |
| Bulimia lifetime | n = 7 | n = 7 | Chi² = 0.25, p = .618 | |
Note: Values are presented in means ± standard deviations or frequencies (n) and percentages (%). Post-hoc Tukey tests are shown for subgroup comparisons. BSL-23 = Borderline Symptom List 23; CTQ = Childhood Trauma Questionnaire; OCD = Obsessive Compulsive Disorder.
Twenty-three BPD patients received psychotropic medication (n = 8; 50% in BPD + PTSD; n = 15; 41% in BPD). Most patients received antidepressants; eight patients took antipsychotics. There were no significant diffrences in medication between BPD and BPD + PTSD. For detailed list of medications in both subgroups see Supplemental Table 1.
2.2. Experimental tasks and instruments
2.2.1. Emotional working memory task
The emotional working memory task (EWMT) was an extended version of the modified Sternberg item recognition task by Krause-Utz and colleagues (Krause-Utz, Elzinga, Oei, Spinhoven, et al., 2014; Krause-Utz et al., 2012, 2020). The present version consisted of 120 trials. At the beginning of each trial, three letters (memoranda, 1000 ms) were presented. After a delay interval (1500 ms), another set of three letters was displayed (probe, 2000 ms). In half of the trials, one of the three memoranda was present in this probe. Participants had to press a ‘yes’ or ‘no’ button indicating whether they had recognized a target letter or not. During the delay interval between the memoranda and the probe, either a fixation cross (distractor-free WM trials) or different distractor stimuli were presented (20 trials each). Distractors were neutral versus (vs.) negative interpersonal scenes from the International Affective Picture System (IAPS) (Bradley & Lang, 2017) as well as neutral vs. angry vs. fearful female facial expressions from the Karolinska Directed Emotional Faces Set (KDEF) (Calvo & Lundqvist, 2008). Participants were instructed to ignore distractors and respond as fast and accurately as possible to the probes, i.e. to inhibit emotional processing in favour of cognitive processing. Order of distractor stimuli and target present/absent trials was pseudorandomized (counter-balanced across subjects). Outcomes were reaction times (RTs) of correct trials and errors. Before and after the task, participants rated their subjective arousal (1 = ‘not at all aroused’ to 10 = ‘extremely aroused’) on a visual analogue scale (Bradley & Lang, 1994). Software Presentation (http://www.neurobs.com/) was used for presenting stimuli and recording data.
2.2.2. Childhood trauma questionnaire
The Childhood Trauma Questionnaire short form (CTQ-SF) (Bernstein et al., 2003) was used to assesses emotional abuse, physical abuse, and sexual abuse, and neglect (5 items each, from 1 = ‘never true’ to 5 = ‘very often true’). The CTQ previously demonstrated good psychometric properties, including good test-retest reliability (Bernstein et al., 2003), also in German samples (Karos, Niederstrasser, Abidi, Bernstein, & Bader, 2014). Internal consistencies (Cronbach’s α) in our sample ranged from α = .89 for physical abuse to α = .94 for emotional abuse as well as sexual abuse.
2.3. Procedure
The study was approved by the local ethics committee. All participants were informed about the background and procedure and that they could terminate the study at any point in time without any negative consequences. After informed consent had been obtained, participants underwent clinical diagnostics and completed the CTQ. They were then invited to a laboratory of the [Central Institute of Mental Health, Germany]. At rest and during the EWMT, electrocardiogram (ECG) data were obtained according to guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Devices of the Biosemi Active Two system with a sampling rate of 1024 Hz were used (Biosemi, Amsterdam, Netherlands, ActiView software; http://www.biosemi.com). Two Ag/AgCl electrodes were placed under the right clavicle and on the left side under the lowest rib. First, participants were instructed to sit still while a 5-min baseline assessment of HRV was conducted (Electrophysiology, 1996). To create a relaxing atmosphere, a soundless 5-min nature film was presented in the background. Then, participants practiced the EWMT (5–10 min), were provided feedback and encouraged to ask questions. While participants performed the task (13–15 min), the experimenter stayed in a separate adjoining room. After the study, some participants were included in a longitudinal randomized-control trial (Krause-Utz et al., 2020). Participants were debriefed, thanked and reimbursed (12€/h).
2.4. Statistical analysis
Software IBM SPSS Statistics 27.0 for Windows was used, with a significance level of p < .05, two-tailed. For significant effects, partial η21 or Cohen’s d2 are reported (Cohen, 2013).
2.4.1. Power calculation
Power calculations were performed for the planned analyses (repeated measure analysis of variance; regression analysis with 3 predictors). With a power of 0.95 (0.05% ß-error probability), a medium to large effect (ƒ = 0.4 and ƒ² = 0.27 respectively) could be detected.
2.4.2. Preprocessing
ECG data were pre-processed using Kubios HRV Finland, http://kubios.uef.fi/ (Tarvainen, Niskanen, Lipponen, Ranta-aho, & Karjalainen, 2014). Recorded data were visually inspected for false or undetected R-waves. Data with technical or physiological artefacts were excluded (n = 6, see above). Spectral analysis was used to calculate high-frequency variations in beat-to-beat intervals (0.15–0.40 Hz). According to established guidelines (Electrophysiology, 1996) for improving robustness of data, HF-HRV measures of the EWMT conditions were averaged and data was analyzed using an autoregressive (AR) model, which is independent of signal length (Di Simplicio et al., 2012; Parati, Saul, Di Rienzo, & Mancia, 1995). Analyses are performed with normalized values.
Raw data of the EWMT was pre-processed by scoring correct trials and errors trials separately. Based on our previous studies, we conceptualized eWM deficits as prolonged RTs of correct trials (Krause-Utz et al., 2012). To control for basic WM, we created separate contrast scores for each distractor condition (angry faces, fearful faces, neutral faces, neutral scenes, and negative scenes, respectively) minus distractor-free trials. Data were averaged to create a single outcome measure for the mediation analysis. Assumptions of normality, linearity, homoscedasticity, and sphericity were tested (using Kolmogorov Smirnov tests, visual inspection of graphic plots etc.). For RTs, five outliers were identified, but Cook’s distance and leverage values suggested no influential cases; data was, therefore, not excluded from the analysis to avoid loss of data and information. Multicollinearity between predictors was unlikely according to tolerance values (>.90) and VIF (<1.1).
2.4.3. Group differences in reaction times and HF-HRV
Group differences in RTs and HF-HRV were tested using two separate repeated measure analyses of covariance (rm-ANCOVAs). In the first analysis (2 × 5 rm-ANCOVA), group (BPD, HC) was the between-subject factor, and the five contrast scores for RTs during each distractor condition minus no distraction were the dependent variables. In the second analysis (2 × 2 rm-ANCOVA), HF-HRV data (rest vs. EWMT) were dependent variables. Mauchly’s test and, if needed, Greenhouse-Geisser-corrections were applied. Equality of variances were checked with Levene’s tests. Since psychotropic medication, especially antidepressants, was found to influence HRV (Krause-Utz, Erol et al., 2019; O’Regan et al., 2015), we included medication status as covariate in all analyses. We categorized medication into no medication, antidepressants, and antipsychotics, as in our previous study (Krause-Utz, Walther et al. 2019).
2.4.4. Subgroup analysis
A subgroup analysis was performed to investigate the influence of comorbid PTSD. The afore-mentioned rm-ANCOVAs were repeated with patients with BPD and PTSD (n = 16), BPD patients (n = 37), and healthy controls (n = 28). Medication status was the covariate.
2.4.5. Linear regression analyses
We performed separate linear regression analyses, to test the ‘total effects’ of childhood trauma and HF-HRV on RTs (i.e. without taking each other’s effect into account). Childhood trauma severity was represented by the sum of the CTQ. In case of significant effects, associations between the CTQ subscales and the outcome measures were evaluated using Spearman Rank correlations.
2.4.6. Mediation analysis
To test our hypotheses that severity of childhood maltreatment has a significant direct effect and a significant indirect effect through HF-HRV on RTs, we used the SPSS PROCESS macro (v3, 4.1), provided by Hayes, and performed a conditional mediation analysis (Hayes & Preacher, 2014). This parametric bootstrapping procedure generates direct and indirect effects of the predicted pathways and allows for conditional modelling, accounting for the group effect (model 14). Regression coefficients were generated based on a bias-corrected confidence interval of 90% and n = 5.000 bootstrapping samples. Severity of childhood trauma (CTQ subscales sum) was defined as predictor (X variable); RTs during distraction (averaged contrast scores) was the outcome variable; HF-HRV during the task was defined as mediator. Group (BPD, HC) was conceptualized as the conditional variable.
3. Results
3.1. Group differences in reaction times
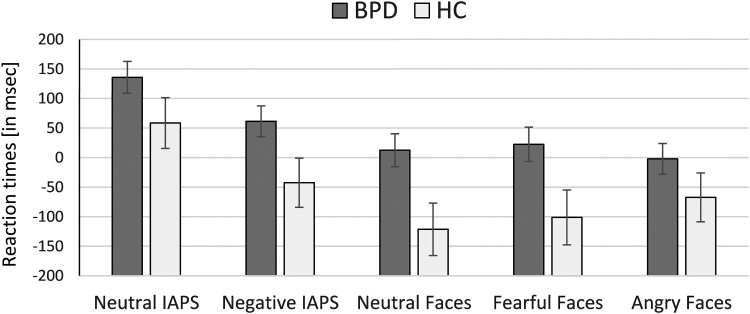
The rm-ANCOVA revealed a significant group effect (F(1,78) = 4.30, p = .041, = .052). Patients with BPD showed overall longer RTs than HC (Figure 1). This group effect was independent of the distractor condition, i.e. the interaction effect was insignificant (F(4,75) = 0.46, p = .764, = .024). There was a significant main effect of distractor condition (F(4,75) = 4.15, p = .004, = .181) with longer RTs after scenes compared to faces (t(80) = 8.43, p < .0001, d = 0.69).
Figure 1.
Reaction times during the task in patients with borderline personality disorder (BPD) and healthy controls (HC).
3.2. Group differences in HF-HRV
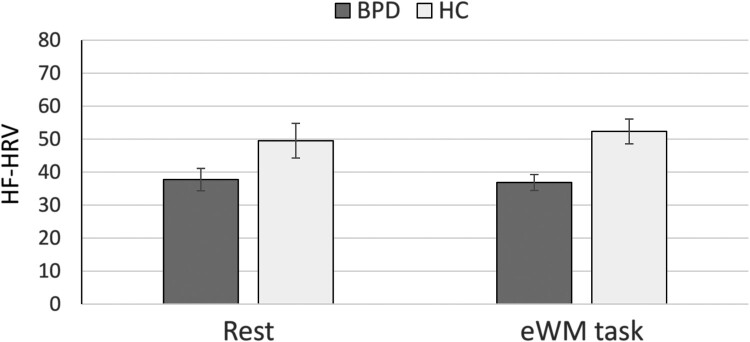
The rm-ANCOVA revealed a significant main effect of group (F(1,76) = 5.22, p = .025, = .064). The other effects were insignificant (main effect of condition: F(1,76) = 0.43, p = .513, = .01; interaction effect: F(4,75) = 0.55, p = .459, = .001). As shown in Figure 2, patients with BPD showed lower HF-HRV during both conditions (rest and EWMT) than HC.
Figure 2.
Heart rate variability in patients with borderline personality disorder (BPD) and healthy controls (HC).
3.3. Effect of medication (covariate)
Medication had no significant effect on RTs (F(1,78) = 1.03, p = .313, = .013). It also did not have a significant effect on HF-HRV (F(1,76) = 2.28, p = .135, = .03).
3.4. Subgroup analyses on the effect of comorbid PTSD
For RTs, no significant effect of PTSD was observed (F(2,77) = 2.28, p = .108, = .056).
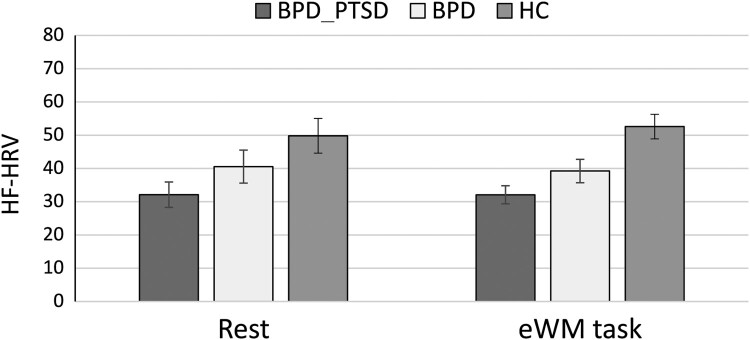
For HRV, the subgroup analysis revealed a significant effect (F(2,75) = 3.76, p = .029, = .10), independent of task condition (F(8,148) = 0.78, p = .606, = .020). Post-hoc Tukey tests indicated significant differences between patients with BPD + PTSD and HC (p = .020), while the other group differences were insignificant (BPD vs. HC: p = .298; BPD vs. BPD + PTSD: p = .250). Patients with BPD + PTSD had the lowest levels of HF-HRV, followed by BPD patients without this comorbidity, followed by HC (see Figure 3).
Figure 3.
Heart rate variability in patients with borderline personality disorder with comorbid posttraumatic stress disorder (BPD + PTSD), patients without comorbid PTSD (BPD), and healthy controls (HC).
3.5. Linear regression analyses
As summarized in Table 3, childhood maltreatment severity (CTQ sum) positively predicted RTs across all distractor conditions (minus no distraction) (Supplemental Figure 1). Follow-up analysis indicated significant positive correlations for all CTQ subscales (all rho ≥ .299, p ≤ .007; Supplemental Table 2).
Table 3.
Results of the multivariate regression analysis for childhood maltreatment (abuse and neglect) severity predicting eWM outcome.
| Reaction times | F(5,55) | p | R² | R²adj | ||
|---|---|---|---|---|---|---|
| Overall model | 3.60 | .007 | .187 | .137 | ||
| F(1,59) | p | t | B | SE | CI (95%) | |
| Negative IAPS | 14.86 | <.001 | 3.86 | 2.952 | 0.766 | [1.420, 4.485] |
| Neutral IAPS | 7.79 | .007 | 2.79 | 2.331 | 0.836 | [0.659, 4.003] |
| Neutral Faces | 4.70 | .034 | 2.17 | 1.920 | 0.886 | [0.147, 3.693] |
| Fearful Faces | 13.58 | <.001 | 3.69 | 3.281 | 0.890 | [1.499, 5.062] |
| Angry Faces | 4.79 | .033 | 2.19 | 1.731 | 0.791 | [0.148, 3.314] |
Note: This table shows results for child maltreatment severity (CTQ sum) predicting reaction times during distraction minus no distraction during the emotional working memory task; CI (95%) = 95% confidence interval.
Severity of childhood maltreatment (CTQ sum) negatively predicted HF-HRV during the EWMT (B = −.158, SE = .075, t = 2.17, p = .039, CI: [−.307, −.009]). Follow-up analyses revealed significant negative correlations for sexual abuse (rho = −.416, p = .003) and physical abuse subscales (rho = −.332, p = .004; all others rho ≤ −.214, p ≥ .095).
3.6. Conditional mediation analysis
The overall regression model, including all predictors and covariates, was significant (F(4,56) = 4.18, p = .005, R² = .230). Childhood maltreatment severity negatively predicted HF-HRV (B = −.158, SE = .075, t = 2.12, p = .039, CI = [−.282, −.033]) and positively predicted RTs during distractors (B = 2.726, SE = 1.029, t = 2.65, p = .011, CI = [1.004, 4.447]). The effects of group and HF-HRV were not significant (both p ≥ .175). There was a significant indirect effect of childhood trauma severity through HF-HRV on RTs in the BPD group (B = −.918, SE = −.426, CI = [−.958, −.061]) but not in HC (B = .099, SE = .460, CI = [−.523, .937]). When controlling for comorbid PTSD, however, this group effect did not reach significance (B = 0.854, SE = 0.566, CI = [−0.376, 1.815]).
4. Discussion
This study investigated reaction times (RTs) as well as HF-HRV during an emotional working memory task (EWMT) in association with childhood maltreatment severity and comorbid PTSD in BPD. Compared to healthy participants, patients with BPD showed prolonged RTs and reduced HF-HRV during the EWMT regardless of distractor condition. Childhood maltreatment severity positively predicted RTs and negatively predicted HF-HRV during this task, when controlling for medication. A significant indirect effect of childhood maltreatment through HF-HRV on RTs was found in BPD but this may be related to comorbid PTSD.
The finding of prolonged RTs after distraction by social cues is in line with earlier findings (Krause-Utz et al., 2012) and partly confirms our first hypothesis. At the same time, we did not find differential effects for angry faces, which were expected to be particularly strong distractors in BPD. Previous studies found a hypersensitivity and hyper-vigilance to anger cues in BPD (Kaiser et al., 2020; Veague & Hooley, 2014). In our study, pictures of neutral interpersonal encounters were associated with longest RTs, probably due to their greater ambiguity. Overall, both neutral and negative interpersonal scenes (IAPS pictures) were associated with longer RTs than faces. This finding is in line with a previous study (Krause-Utz, Elzinga, Oei, Spinhoven, et al., 2014) and suggests that naturalistic scenes of interpersonal encounters are more complex and salient than faces, and therefore, draw on more attentional resources (see also Sabatinelli et al., 2011).
No significant decrease in HF-HRV during the EWMT as compared to resting-state was found, which resembles earlier findings of studies using emotion regulation tasks (Dixon-Gordon et al., 2011; Fitzpatrick & Kuo, 2016; Krause-Utz, Walther, et al., 2019; Kuo et al., 2016; Metcalfe et al., 2017; Svaldi et al., 2012). At the same time, we observed significant group differences in HF-HRV. Patients with BPD showed lower HRV during rest as well as during the EMWT than healthy participants. However, a subgroup analysis indicated that this was associated with comorbid PTSD. While this is in line with an earlier study (Krause-Utz, Walther et al., 2019), the present subgroup of PTSD patients was relatively small and reported significantly higher levels of childhood abuse and neglect than BPD patients without PTSD. Therefore, it remains unclear whether lower HF-HRV in patients with BPD + PTSD is due to higher levels of childhood trauma in this group or due to the presence of PTSD. We did not include a group of PTSD patients without BPD. Thus, the present findings may be specific to a subgroup with overall greater psychosocial impairments. Future research should include larger subsamples and respective control groups to these corroborate findings. While previous studies found a significant effect of medication status on HRV (Krause-Utz, Walther, et al., 2019; O’Regan et al., 2015), this was not observed in the present study. Since we cannot rule out that the type or dosage of drugs influenced the results, future studies are needed to investigate if they can be confirmed in an entirely unmedicated sample.
Across groups, severity of childhood maltreatment (abuse, neglect) predicted longer RTs and lower HF-HRV during the EWMT. Severe childhood abuse and neglect can lead to a hypervigilance towards threatening social cues, such as angry faces (Kaiser et al., 2016; Pollak et al., 2005; Seitz et al., 2021), which may result in rejection sensitivity (Downey & Feldman, 1996). Our findings suggest that this attentional bias may not necessarily be limited to threatening social cues but may generally interfere with the processing of interpersonal stimuli. This may in turn interfere with WM resources. Since WM plays a crucial role in goal-directed behaviour and emotional self-regulation (Koch et al., 2018) our findings may have important clinical implications. Paying attention to a history of childhood trauma may be necessary to understand difficulties in emotion regulation, especially in interpersonal situations. Seemingly neutral interpersonal cues may be perceived as disturbing and interfere with basic executive functions that are important to maintain goal-directed behaviour. Psychoeducation might help increasing the awareness on this relationship. There is also some evidence that eWM training with socio-emotional distractors (e.g. faces, scenes) may have beneficial effects on emotion regulation capacities in healthy individuals (Schweizer et al., 2019) and in patients with BPD (Krause-Utz et al., 2020). Longitudinal studies with larger groups are needed to corroborate our findings. Using additional experimental tasks (e.g. emotion classification and emotion regulation tasks) may help to further deepen the understanding of dysfunctional emotion-related information processing. Among others, anticipating regulatory processes may play an important role in this context (Kaiser et al., 2016b; Seitz et al., 2021).
While the use of a well-established paradigm and well-characterized groups are strengths of our study, findings should be regarded as preliminary, given the relatively small sample sizes. Since childhood maltreatment was assessed retrospectively, we cannot rule out that participants’ responses were biased, due to a lack of awareness, minimizing, or social desirability. Most healthy participants in our studies reported no or minimal childhood maltreatment. PTSD was assessed using the SCID, which may not sufficiently capture the diagnosis, especially more complex forms of PTSD. Future research should use additional scales to verify PTSD diagnosis. As childhood trauma severity and PTSD comorbidity were confounded in our subgroup of BPD + PTSD patients, future research needs to include subgroups with similar levels of childhood trauma severity. Furthermore, we focused on HF-HRV, because we were mainly interested in associations between parasympathetic activity, traumatic stress, and eWM performance in BPD. This limits the comparisons of our findings with studies using other HRV measures (Carr, de Vos, & Saunders, 2018). Lastly, we only included female participants and findings cannot be generalized to male patients.
5. Conclusion
Our study suggests that childhood maltreatment severity is associated with increased attention (prolonged RTs) and lower HF-HRV (parasympathetic activation) during an EWMT. Hypervigilance to social cues may be more pronounced in patients with BPD, regardless of comorbid PTSD, while altered parasympathetic activation seems to be related to the co-occurrence of both disorders or higher levels of traumatic stress. Maintaining WM during distressing situations is crucial for goal-directed behaviour, emotional and behavioural self-regulation, academic achievement, and establishing meaningful relationships. Given this crucial role of eWM, more research is needed to understand variables that underlie eWM deficits in BPD. This will help identify subgroups (e.g. those with a history of childhood maltreatment) who may profit from eWM training, aimed at improving emotion regulation.
Supplementary Material
Acknowledgements
The authors thank all participants of this study for their crucial contribution. We thank Rachel Frost, Anna Ternies, Charlotte Harland, Majbritt Jepsen, and Sophia Scholtes for their contribution to the pre-processing of the data. All subjects have given their written informed consent. The study protocol has been approved by the local Ethical committee. All co-authors gave consent for publication. AKU, JCW, and SL designed the study and its rationale. AKU designed the structure of the paper and drafted its first version; JCW recruited participants and collected data. AKU, AK, and AT performed statistical analyses and prepared the results. SL, WH, CS, and MB provided input throughout each stage of writing. All authors contributed to the final version of the paper.
Notes
≤ .039 representing small effects, = .04–.139 mediate effects and ≥ .14 large effects.
d = 0.20 representing small effects, d = 0.50 mediate effects, d = .0.80 large effects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
According to European law (GDPR), data containing potentially identifying or sensitive patient information are restricted; our data involving clinical participants are not freely available in the manuscript, supplemental files, or in a public repository. Data access can be requested on reasonable demand via the corresponding author.
References
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Fifth edition.
- Appelhans, B. M., & Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. doi: 10.1037/1089-2680.10.3.229 [DOI] [Google Scholar]
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., … Zule, W. (2003). Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect, 27(2), 169–190. doi: 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Bohus, M., Kleindienst, N., Limberger, M. F., Stieglitz, R.-D., Domsalla, M., Chapman, A. L., … Wolf, M. (2009). The short version of the Borderline Symptom List (BSL-23): Development and initial data on psychometric properties. Psychopathology, 42(1), 32–39. doi: 10.1159/000173701 [DOI] [PubMed] [Google Scholar]
- Bohus, M., Stoffers-Winterling, J., Sharp, C., Krause-Utz, A., Schmahl, C., & Lieb, K. (2021). Borderline personality disorder. [DOI] [PubMed]
- Bradley, M. M., & Lang, P. J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. doi: 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Bradley, M. M., & Lang, P. J. (2017). International affective picture system. In Zeigler-Hill V., & Shackelford T. K. (Eds.), Encyclopedia of personality and individual differences (pp. 1–4). Springer International Publishing. doi: 10.1007/978-3-319-28099-8_42-1 [DOI] [Google Scholar]
- Cackowski, S., Neubauer, T., & Kleindienst, N. (2016). The impact of posttraumatic stress disorder on the symptomatology of borderline personality disorder. Borderline Personality Disorder and Emotion Dysregulation, 3(1), 7. doi: 10.1186/s40479-016-0042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo, M. G., & Lundqvist, D. (2008). Facial expressions of emotion (KDEF): Identification under different display-duration conditions. Behavior Research Methods, 40(1), 109–115. doi: 10.3758/BRM.40.1.109 [DOI] [PubMed] [Google Scholar]
- Carr, O., de Vos, M., & Saunders, K. E. A. (2018). Heart rate variability in bipolar disorder and borderline personality disorder: A clinical review. Evidence Based Mental Health, 21(1), 23–30. doi: 10.1136/eb-2017-102760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Fernando, S., Beblo, T., Schlosser, N., Terfehr, K., Otte, C., Löwe, B., … Wingenfeld, K. (2014). The impact of self-reported childhood trauma on emotion regulation in borderline personality disorder and major depression. Journal of Trauma & Dissociation, 15(4), 384–401. doi: 10.1080/15299732.2013.863262 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Routledge. doi: 10.4324/9780203771587 [DOI] [Google Scholar]
- Denny, B. T., & Ochsner, K. N. (2014). Behavioral effects of longitudinal training in cognitive reappraisal. Emotion, 14(2), 425–433. doi: 10.1037/a0035276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio, M., Costoloni, G., Western, D., Hanson, B., Taggart, P., & Harmer, C. J. (2012). Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychological Medicine, 42(8), 1775–1783. doi: 10.1017/S0033291711002479 [DOI] [PubMed] [Google Scholar]
- Dixon-Gordon, K. L., Chapman, A. L., Lovasz, N., & Walters, K. (2011). Too upset to think: The interplay of borderline personality features, negative emotions, and social problem solving in the laboratory. Personality Disorders: Theory, Research, and Treatment, 2(4), 243–260. doi: 10.1037/a0021799 [DOI] [PubMed] [Google Scholar]
- Domes, G., Czieschnek, D., Weidler, F., Berger, C., Fast, K., & Herpertz, S. C. (2008). Recognition of facial affect in borderline personality disorder. Journal of Personality Disorders, 22(2), 135–147. doi: 10.1521/pedi.2008.22.2.135 [DOI] [PubMed] [Google Scholar]
- Downey, G., & Feldman, S. I. (1996). Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology, 70(6), 1327–1343. doi: 10.1037/0022-3514.70.6.1327 [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer, U. W., Kuo, J., Schlotz, W., Kleindienst, N., Rosenthal, M. Z., Detterer, L., … Bohus, M. (2008). Distress and affective dysregulation in patients with borderline personality disorder: A psychophysiological ambulatory monitoring study. Journal of Nervous & Mental Disease, 196(4), 314–320. doi: 10.1097/NMD.0b013e31816a493f [DOI] [PubMed] [Google Scholar]
- Electrophysiology, T. F. of the E. S . (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93(5), 1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, S., & Kuo, J. R. (2016). The impact of stimulus arousal level on emotion regulation effectiveness in borderline personality disorder. Psychiatry Research, 241, 242–248. doi: 10.1016/j.psychres.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Glaesmer, H., Schulz, A., Häuser, W., Freyberger, H., Brähler, E., & Grabe, H.-J. (2013). Der childhood trauma screener (CTS)—entwicklung und validierung von schwellenwerten zur klassifikation. Psychiatrische Praxis, 40(04), 220–226. doi: 10.1055/s-0033-1343116 [DOI] [PubMed] [Google Scholar]
- Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. doi: 10.1016/j.biopsycho.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Gunderson, J. G., Herpertz, S. C., Skodol, A. E., Torgersen, S., & Zanarini, M. C. (2018). Borderline personality disorder. Nature Reviews Disease Primers, 4(1), 18029. doi: 10.1038/nrdp.2018.29 [DOI] [PubMed] [Google Scholar]
- Hansen, A. L., Johnsen, B. H., & Thayer, J. F. (2003). Vagal influence on working memory and attention. International Journal of Psychophysiology, 48(3), 263–274. doi: 10.1016/S0167-8760(03)00073-4 [DOI] [PubMed] [Google Scholar]
- Hayes, A. F., & Preacher, K. J. (2014). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67(3), 451–470. doi: 10.1111/bmsp.12028 [DOI] [PubMed] [Google Scholar]
- Holzman, J. B., & Bridgett, D. J. (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience & Biobehavioral Reviews, 74, 233–255. doi: 10.1016/j.neubiorev.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Kaiser, D., Jacob, G. A., Domes, G., & Arntz, A. (2016). Attentional bias for emotional stimuli in borderline personality disorder: A meta-analysis. Psychopathology, 49(6), 383–396. doi: 10.1159/000448624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, D., Jacob, G. A., van Zutphen, L., Siep, N., Sprenger, A., Tuschen-Caffier, B., … Domes, G. (2020). Patients with borderline personality disorder and comorbid PTSD show biased attention for threat in the facial dot-probe task. Journal of Behavior Therapy and Experimental Psychiatry, 67, 101437. doi: 10.1016/j.jbtep.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Karos, K., Niederstrasser, N., Abidi, L., Bernstein, D. P., & Bader, K. (2014). Factor structure, reliability, and known groups validity of the German version of the childhood trauma questionnaire (short-form) in Swiss patients and nonpatients. Journal of Child Sexual Abuse, 23(4), 418–430. doi: 10.1080/10538712.2014.896840 [DOI] [PubMed] [Google Scholar]
- Kemp, A. H., & Quintana, D. S. (2013). The relationship between mental and physical health: Insights from the study of heart rate variability. International Journal of Psychophysiology, 89(3), 288–296. doi: 10.1016/j.ijpsycho.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Kemp, A. H., Quintana, D. S., Felmingham, K. L., Matthews, S., & Jelinek, H. F. (2012). Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS One, 7(2), e30777. doi: 10.1371/journal.pone.0030777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, S. B. J., Mars, R. B., Toni, I., & Roelofs, K. (2018). Emotional control, reappraised. Neuroscience & Biobehavioral Reviews, 95, 528–534. doi: 10.1016/j.neubiorev.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Koenig, J., Kemp, A. H., Feeling, N. R., Thayer, J. F., & Kaess, M. (2016). Resting state vagal tone in borderline personality disorder: A meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 18–26. doi: 10.1016/j.pnpbp.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Krause-Utz, A., Elzinga, B. M., Oei, N. Y. L., Paret, C., Niedtfeld, I., Spinhoven, P., … Schmahl, C. (2014). Amygdala and dorsal anterior cingulate connectivity during an emotional working memory task in borderline personality disorder patients with interpersonal trauma history. Frontiers in Human Neuroscience, 8. doi: 10.3389/fnhum.2014.00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz, A., Elzinga, B. M., Oei, N. Y. L., Spinhoven, P., Bohus, M., & Schmahl, C. (2014). Susceptibility to distraction by social cues in borderline personality disorder. Psychopathology, 47(3), 148–157. doi: 10.1159/000351740 [DOI] [PubMed] [Google Scholar]
- Krause-Utz, A., Erol, E., Brousianou, A. V., Cackowski, S., Paret, C., Ende, G., & Elzinga, B. (2019). Self-reported impulsivity in women with borderline personality disorder: The role of childhood maltreatment severity and emotion regulation difficulties. Borderline Personality Disorder and Emotion Dysregulation, 6(1), 6. doi: 10.1186/s40479-019-0101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz, A., Oei, N. Y. L., Niedtfeld, I., Bohus, M., Spinhoven, P., Schmahl, C., & Elzinga, B. M. (2012). Influence of emotional distraction on working memory performance in borderline personality disorder. Psychological Medicine, 42(10), 2181–2192. doi: 10.1017/S0033291712000153 [DOI] [PubMed] [Google Scholar]
- Krause-Utz, A., Walther, J.-C., Lis, S., Schmahl, C., & Bohus, M. (2019). Heart rate variability during a cognitive reappraisal task in female patients with borderline personality disorder: The role of comorbid posttraumatic stress disorder and dissociation. Psychological Medicine, 49(11), 1810–1821. doi: 10.1017/S0033291718002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz, A., Walther, J.-C., Schweizer, S., Lis, S., Hampshire, A., Schmahl, C., & Bohus, M. (2020). Effectiveness of an emotional working memory training in borderline personality disorder: A proof-of-principle study. Psychotherapy and Psychosomatics, 89(2), 122–124. doi: 10.1159/000504454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz, A., Winter, D., Schriner, F., Chiu, C.-D., Lis, S., Spinhoven, P., … Elzinga, B. M. (2018). Reduced amygdala reactivity and impaired working memory during dissociation in borderline personality disorder. European Archives of Psychiatry and Clinical Neuroscience, 268(4), 401–415. doi: 10.1007/s00406-017-0806-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, J. R., Fitzpatrick, S., Metcalfe, R. K., & McMain, S. (2016). A multi-method laboratory investigation of emotional reactivity and emotion regulation abilities in borderline personality disorder. Journal of Behavior Therapy and Experimental Psychiatry, 50, 52–60. doi: 10.1016/j.jbtep.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Laborde, S., Furley, P., & Schempp, C. (2015). The relationship between working memory, reinvestment, and heart rate variability. Physiology & Behavior, 139, 430–436. doi: 10.1016/j.physbeh.2014.11.036 [DOI] [PubMed] [Google Scholar]
- Lazarus, S. A., Cheavens, J. S., Festa, F., & Zachary Rosenthal, M. (2014). Interpersonal functioning in borderline personality disorder: A systematic review of behavioral and laboratory-based assessments. Clinical Psychology Review, 34(3), 193–205. doi: 10.1016/j.cpr.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Lehrl, S., Triebig, G., & Fischer, B. (1995). Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurologica Scandinavica, 91(5), 335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x [DOI] [PubMed] [Google Scholar]
- Lobbestael, J., Leurgans, M., & Arntz, A. (2011). Inter-rater reliability of the structured clinical interview for DSM-IV Axis I disorders (SCID I) and Axis II disorders (SCID II). Clinical Psychology & Psychotherapy, 18(1), 75–79. doi: 10.1002/cpp.693 [DOI] [PubMed] [Google Scholar]
- Loranger, A. W. (1994). The international personality disorder examination: The world health organization/alcohol, drug abuse, and mental health administration international pilot study of personality disorders. Archives of General Psychiatry, 51(3), 215. doi: 10.1001/archpsyc.1994.03950030051005 [DOI] [PubMed] [Google Scholar]
- Mccraty, R., & Shaffer, F. (2015). Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global Advances in Health and Medicine, 4(1), 46–61. doi: 10.7453/gahmj.2014.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe, R. K., Fitzpatrick, S., & Kuo, J. R. (2017). A laboratory examination of emotion regulation skill strengthening in borderline personality disorder. Personality Disorders: Theory, Research, and Treatment, 8(3), 237–246. doi: 10.1037/per0000156 [DOI] [PubMed] [Google Scholar]
- Meyer, P.-W., Müller, L. E., Zastrow, A., Schmidinger, I., Bohus, M., Herpertz, S. C., & Bertsch, K. (2016). Heart rate variability in patients with post-traumatic stress disorder or borderline personality disorder: Relationship to early life maltreatment. Journal of Neural Transmission, 123(9), 1107–1118. doi: 10.1007/s00702-016-1584-8 [DOI] [PubMed] [Google Scholar]
- O’Regan, C., Kenny, R. A., Cronin, H., Finucane, C., & Kearney, P. M. (2015). Antidepressants strongly influence the relationship between depression and heart rate variability: Findings from The Irish Longitudinal Study on Ageing (TILDA). Psychological Medicine, 45(3), 623–636. doi: 10.1017/S0033291714001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N., & Gross, J. J. (2008). Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17(2), 153–158. doi: 10.1111/j.1467-8721.2008.00566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagura, J., Stein, M. B., Bolton, J. M., Cox, B. J., Grant, B., & Sareen, J. (2010). Comorbidity of borderline personality disorder and posttraumatic stress disorder in the U.S. population. Journal of Psychiatric Research, 44(16), 1190–1198. doi: 10.1016/j.jpsychires.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati, G., Saul, J. P., Di Rienzo, M., & Mancia, G. (1995). Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation: A critical appraisal. Hypertension, 25(6), 1276–1286. doi: 10.1161/01.HYP.25.6.1276 [DOI] [PubMed] [Google Scholar]
- Pollak, S. D., Vardi, S., Putzer Bechner, A. M., & Curtin, J. J. (2005). Physically abused children’s regulation of attention in response to hostility. Child Development, 76(5), 968–977. doi: 10.1111/j.1467-8624.2005.00890.x [DOI] [PubMed] [Google Scholar]
- Porter, C., Palmier-Claus, J., Branitsky, A., Mansell, W., Warwick, H., & Varese, F. (2020). Childhood adversity and borderline personality disorder: A meta-analysis. Acta Psychiatrica Scandinavica, 141(1), 6–20. doi: 10.1111/acps.13118 [DOI] [PubMed] [Google Scholar]
- Sabatinelli, D., Fortune, E. E., Li, Q., Siddiqui, A., Krafft, C., Oliver, W. T., … Jeffries, J. (2011). Emotional perception: Meta-analyses of face and natural scene processing. NeuroImage, 54(3), 2524–2533. doi: 10.1016/j.neuroimage.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Sadikaj, G., Russell, J. J., Moskowitz, D. S., & Paris, J. (2010). Affect dysregulation in individuals with borderline personality disorder: Persistence and interpersonal triggers. Journal of Personality Assessment, 92(6), 490–500. doi: 10.1080/00223891.2010.513287 [DOI] [PubMed] [Google Scholar]
- Sakaki, M., Yoo, H. J., Nga, L., Lee, T.-H., Thayer, J. F., & Mather, M. (2016). Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage, 139, 44–52. doi: 10.1016/j.neuroimage.2016.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo, P., Reinhard, I., Mussgay, L., Steil, R., Sawitzki, G., Klein, C., … Ebner-Priemer, U. W. (2014). Specificity of affective instability in patients with borderline personality disorder compared to posttraumatic stress disorder, bulimia nervosa, and healthy controls. Journal of Abnormal Psychology, 123(1), 258–272. doi: 10.1037/a0035619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, M., & Schwerdtfeger, A. (2020). Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: A meta-analysis. Psychological Medicine, 50(12), 1937–1948. doi: 10.1017/S003329172000207X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, S., Satpute, A. B., Atzil, S., Field, A. P., Hitchcock, C., Black, M., … Dalgleish, T. (2019). The impact of affective information on working memory: A pair of meta-analytic reviews of behavioral and neuroimaging evidence. Psychological Bulletin, 145(6), 566–609. doi: 10.1037/bul0000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz, K. I., Leitenstorfer, J., Krauch, M., Hillmann, K., Boll, S., Ueltzhoeffer, K., … Bertsch, K. (2021). An eye-tracking study of interpersonal threat sensitivity and adverse childhood experiences in borderline personality disorder. Borderline Personality Disorder and Emotion Dysregulation, 8(1), 2. doi: 10.1186/s40479-020-00141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. doi: 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi, J., Dorn, C., Matthies, S., & Philipsen, A. (2012). Effects of suppression and acceptance of sadness on the urge for non-suicidal self-injury and self-punishment. Psychiatry Research, 200(2–3), 404–416. doi: 10.1016/j.psychres.2012.06.030 [DOI] [PubMed] [Google Scholar]
- Tarvainen, M. P., Niskanen, J.-P., Lipponen, J. A., Ranta-aho, P. O., & Karjalainen, P. A. (2014). Kubios HRV – Heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. doi: 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Trull, T. J., Jahng, S., Tomko, R. L., Wood, P. K., & Sher, K. J. (2010). Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. Journal of Personality Disorders, 24(4), 412–426. doi: 10.1521/pedi.2010.24.4.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veague, H. B., & Hooley, J. M. (2014). Enhanced sensitivity and response bias for male anger in women with borderline personality disorder. Psychiatry Research, 215(3), 687–693. doi: 10.1016/j.psychres.2013.12.045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
According to European law (GDPR), data containing potentially identifying or sensitive patient information are restricted; our data involving clinical participants are not freely available in the manuscript, supplemental files, or in a public repository. Data access can be requested on reasonable demand via the corresponding author.