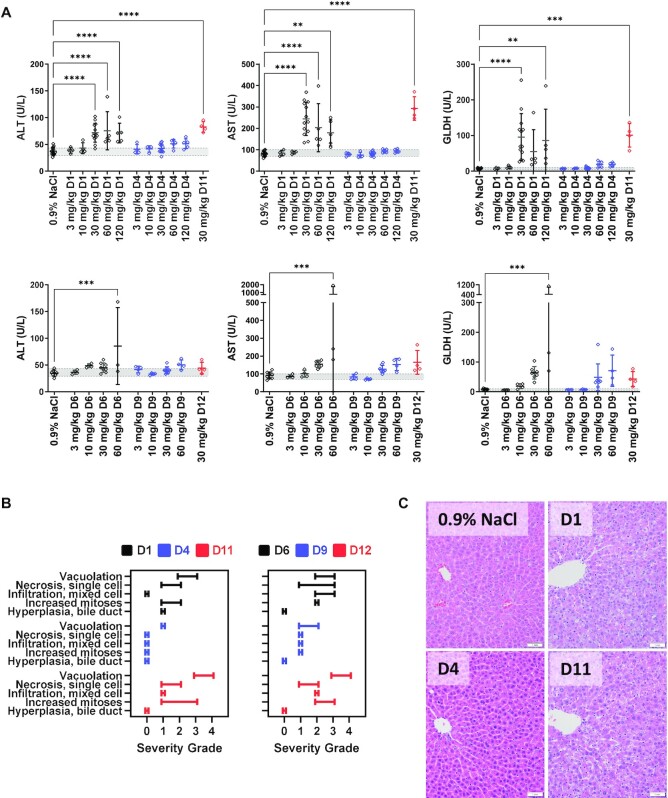
Figure 2.
Selected clinical pathology parameters and microscopic liver findings measured in rats (n = 4–5) following three weekly doses of parent, GNA- or DNA-modified GalNAc–siRNAs targeting Ttr or Hao1. (A) Measured liver enzymes from serum that was collected 24 h after final dose. Data were collected from three different studies; D1, D4, D6 and D9 were evaluated in a single study at 3, 10 and 30 mg/kg, and two subsequent studies evaluated D1 and D4 or D6 and D9 at 30, 60 and 120 mg/kg, and D11 or D12 at 30 mg/kg. Controls and the overlapping 30 mg/kg groups were combined and plotted above. (B) Summary of the range of microscopic liver findings based on severity grade. (C) Microscopic liver findings in rats following three weekly doses of 30 mg/kg with the indicated siRNAs targeting Ttr compared to the 0.9% NaCl control. Livers were collected 24 h post-final dose for analysis and sections were stained with H&E.