Abstract
Radiation therapy (RT) has dramatically improved cancer survival, leading to several inevitable complications. Unintentional irradiation of the heart can lead to radiation-induced heart disease (RIHD), including cardiomyopathy, pericarditis, coronary artery disease, valvular heart disease, and conduction system abnormalities. Furthermore, the development of RIHD is aggravated with the addition of chemotherapy. The screening, diagnosis, and follow-up for RIHD in patients who have undergone RT are described by the consensus guidelines from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). There is compelling evidence that chest RT can increase the risk of heart disease. Although the prevalence and severity of RIHD are likely to be reduced with modern RT techniques, the incidence of RIHD is expected to rise in cancer survivors who have been treated with old RT regimens. However, there remains a gap between guidelines and clinical practice. Currently, therapeutic modalities followed in the treatment of RIHD are similar to the non-irradiated population. Preventive measures mainly reduce the radiation dose and radiation volume of the heart. There is no concrete evidence to endorse the preventive role of statins, angiotensin-converting enzyme inhibitors, and antioxidants. This review summarizes the current evidence of RIHD subtypes and risk factors and suggests screening regimens, diagnosis, treatment, and preventive approaches.
Keywords: Radiation-induced abnormalities, Radiotherapy, Antioxidants, Cardiomyopathies, Coronary artery disease, Inflammation, Oxidative stress
Introduction
Owing to the increased incidence and surveillance of tumors, radiation therapy (RT) has become a vital component of oncological therapeutics [1]. RT has dramatically improved the survival and longevity of patients with breast and thoracic malignancy; however, collateral damage to nearby structures has led to inevitable complications that compromise the overall treatment yield. Radiation-induced heart disease (RIHD) has gained more recognition as a significant source of morbidity and mortality in cancer survivors [2-5]. Cardiac dysfunction due to radiation comprises a spectrum of acute and chronic manifestations of heart disease—pericarditis, cardiomyopathy, coronary artery disease (CAD), valvular heart disease (VHD), and cardiac conduction abnormalities [2]. These complications could arise in patients undergoing RT for various malignancies close to the heart, such as breast, lung, or esophageal cancer, as well as thymoma and mediastinal lymphoma [5].
Cardiovascular complications constitute the most common non-malignant cause of death amongst cancer survivors who received RT [6]. The risk of such complications is further augmented by the combined contribution of chemotherapy and cardiovascular risk factors, including diabetes, hypertension, obesity, and dyslipidemia. The estimated incidence of RIHD was reported as 10%–30% after 5–10 years post-treatment, and it varies with the time of RT and type of malignancy [6]. For example, there is a 1.7- to 2-fold increase in cardiovascular mortality amongst radiated patients, and the risk further increases to 7.2-fold in those who received radiation before the 1970s [7]. In addition, the incidence of RIHD is higher in lymphoma patients as compared to breast cancer patients, approaching 49.5%–54.5%, with a variable incidence of each subtype of heart disease within each cancer population (0.5%–37% for breast cancer patients vs. 11%–31% for lymphoma patients) [8]. The morbidity of RIHD in other thoracic cancers is poorly reflected by short survival and poor follow-up time. According to a meta-analysis, RT for lung cancer increases the risk for cardiac-specific mortality by nearly 30%. A higher mean heart dose (MHD) of ≥5 Gy and 30 Gy and the irradiated heart volume were risk factors for the development of RIHD [9]. As a result, RIHD has been a major limiting factor to consider when deciding on radiation doses, thereby compromising the efficacy of RT for thoracic tumors [10,11].
Jacob et al. [12] suggested that the MHD is insufficient to predict the individual patient dose to the left ventricle (LV) and coronary arteries, especially the left anterior descending artery (LAD). They further suggested that it would be necessary to contemplate the distribution of doses within these cardiac substructures, rather than just the MHD, to generate precise RT-induced cardiotoxicity studies. Vivekanandan et al. [13] assessed the association between all-cause death rate, cardiac radiation doses, and electrocardiographic changes in 78 patients with locally advanced non-small cell lung cancer (NSCLC) treated in the IDEAL-CRT (a trial of isotoxically escalated concurrent chemoradiation delivering tumor doses of 63–73 Gy), and they found that conduction or ischemic/pericarditis-like changes on electrocardiogram (ECG) at 6 months as well as receiving higher heart or left atrial wall volumes of 63–69 Gy were associated with higher mortality.
The current understanding of RIHD is based on data from cancer survivors who had received RT almost three decades ago, wherein the techniques were less advanced with a broad area of exposure and higher radiation dose [14]. However, current advances in radiotherapeutic technology have reduced the morbidity of RIHD, yet the risk is not eliminated [15,16]. Therefore, surveillance and screening programs utilizing different cardiac imaging modalities have been suggested by the expert consensus guidelines from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). The present review article summarizes the common classification and risk factors of RIHD and further outlines the currently suggested approach for screening, diagnosis, management, and prevention.
Common Cardiovascular Manifestations of RIHD
1. Pericardial disease
Pericardial disease is the most common manifestation of RIHD. It can be divided into early disease, which occurs within days to months post-RT manifesting as acute or exudative pericarditis, and late disease, which may develop months to years later and includes chronic, fibrinous, and constrictive pericarditis [17,18]. The incidence of pericardial disease in patients who received mediastinal radiation is estimated at around 70%, and constrictive pericarditis is observed in up to 20% of patients within 2 years following irradiation [6,19]. However, this risk has been significantly reduced in the last decades to 6%–10%, yet it remains the most common complication of RIHD [8,20-22]. The risk of pericardial disease correlates positively with the radiation dose, with a 5-fold increase in risk with every 10 Gy rise in radiation dose [8,23]. A study showed that 21% of patients with Hodgkin's lymphoma who received radiation doses >35 Gy developed pericardial thickening. In addition, 36% of patients with esophageal cancer who received a dose of 1.8–2.0 Gy per day for 6 weeks developed pericardial effusion [18]. Therefore, dose-volume metrics from the whole heart are frequently incorporated in RT planning and predicting the risk of pericardial disease. However, a recent study by Niedzielski et al. [24] developed a net logistical regression model that considered cardiac substructure dose in predicting pericardial effusion in patients with NSCLC. Results showed that incorporating cardiac substructure dose to whole heart dose toxicity models had superior predictive performance. Left atrium (LA) dose was the most recurring predictor of higher-performing models.
Echocardiography is considered the first line in evaluating patients with radiation-induced pericardial disease, and it can detect pericardial thickening, surrounding effusions, and hemodynamic effects of constrictive pericarditis and pericardial tamponade [7]. Cardiac computerized tomography (CT) and cardiac magnetic resonance (CMR) can also be complementary methods for further delineating inflammation, edema, and fibrosis [6]. Standard management is similar to other forms of pericarditis. Diuretics are used for symptomatic constrictive pericarditis, with pericardiectomy being the definitive management. Those patients had the worst outcome amongst all other causes of constrictive pericarditis, with a 5-year survival rate reaching only 12% [19].
2. Valvular heart disease
RT has also been linked to VHD. Chest radiation leads to activation of fibroblasts and release of fibroblast growth factor, which leads to fibrosis, with or without calcification [25]. Damage is not limited to the cusps only but extends to the valvular annulus, supra- and sub-valvular apparatus, making valvular repair more challenging [18]. Radiation-induced VHD (RIVHD) is observed in nearly 81% of cancer survivors [26]. The incubation period varies, with the incidence of 26% at 10 years rising to 60% at 20 years [19]. Therefore, all patients who received mediastinal RT should have echocardiography after 10 years, with different follow-up intervals depending on valve pathology [25]. The incidence remains higher in patients who received mediastinal radiation for Hodgkin's lymphoma (2%–37%) compared to breast cancer (0.2%–4.2%), this difference and variation in the incidence between studies may be related to differences in study design controls and inherent bias, in addition to the limited number of studies that looked into RIVHD among patients with breast cancer [25]. In addition, there is a stepwise increase in the risk of RIVHD with radiation doses >30 Gy [8,25,27]. Furthermore, the incidence was higher in patients with left breast cancer (LBC) as compared to right breast cancer (RBC) [22]. Left-sided valves are usually more affected, with the most common abnormality being aortic regurgitation, followed by mitral regurgitation. Aortic stenosis, as well as tricuspid and pulmonary regurgitation, are less common but reported manifestations of RIVHD [7,18,28]. Similar to pericardial disease, prediction models have utilized cardiac substructure exposure percentage and dose to predict the occurrence of valvular disease. Cella et al. [29,30] showed that the risk of mitral and aortic valve disease increased if a larger volume of the LA and LV received doses >25–30 Gy. Echocardiography remains the cornerstone of diagnosis for its ability to detect anatomical and functional changes in the valves and their effect on the ventricles [7,25]. With disease progression, patients may require interventional or surgical valve replacement. The standardized incidence ratio for valve surgery compared with an age-matched and sex-matched normal population was 9.2 (95% confidence interval, 8.1–10.3) [25]. However, rates of mortality and adverse events were higher following both interventional and surgical valve replacement in patients with RIVHD than in controls [19,31-33].
3. Conduction system abnormalities
The development of conduction abnormalities is a rare complication of thoracic radiation, mediated by direct radiation-induced inflammatory or ischemic insults, leading to fibrosis. An overall incidence rate of 4%–5% of conduction disturbances was reported, and 75% of long-term cancer survivors with mediastinal radiation were found to have an ECG abnormality [18,19]. These abnormalities usually appear within 2 months of completing RT and involve any of the structures of the cardiac electrical system [8,34]. The most common manifestation is infranodal and right bundle branch block, likely due to its anterior location and direct susceptibly to radiation [18]. However, sinoatrial node dysfunction has also been reported after stereotactic ablative RT (SABR). A retrospective study, which analyzed 13 patients who received thoracic SABR for early-stage lung cancer, found that one patient developed symptomatic sick sinus syndrome mandating pacemaker placement at 6 months of therapy, with her age (83 years) and mean sinoatrial node radiation dose of 40 Gy reported as the third-highest amongst her cohort [35]. Radiation was also associated with a higher prevalence of prolonged QTc, supraventricular premature complexes, and ventricular tachycardia, particularly in children and young adults [36]. About 70% of radiation-related ECG abnormalities are reversible after six months of RT. However, high-grade AV blocks causing complete heart block and permanent pacemaker implantation were also reported [8,36,37]. The effect of cardiac substructure dosimetry on the incidence of late arrhythmias was also studied by Bates et al. [38] showing that a mean radiation dose >10 Gy to the whole heart was associated with an increased risk of arrhythmias. Interestingly, a low RT dose (5–9.9 Gy) to the right coronary artery (RCA) specifically increased the development of cardiac arrhythmias. Radiation-induced arrhythmias are usually handled similar to their traditional counterparts, with ECG and ambulatory Holter monitoring for detection and diagnosis. Pacemakers and defibrillators are inserted when indicated, in which a subpectoral approach can be considered in cases of extensive cutaneous fibrosis [19].
4. Cardiomyopathy
Cardiomyopathy and heart failure (HF) are other potential complications of chest radiation. Acute myocardial toxic effects of RT, mediated by inflammation, myocardial dysfunction, and repolarization abnormalities, are rare [7]. The clinical manifestations of RT-related cardiac fibrosis present late with an incubation period of around 10 years, particularly in those exposed to >30 Gy, ultimately leading to symptoms of HF [19,39]. The estimated prevalence of radiation-induced cardiomyopathy in literature is >10%, with an increased incidence of HF compared to the general population (SIR of 4.9 with 25.6 excess cases per 10,000 patients/year) [18,19]. In addition, a radiation dose-response relationship has been reported in a recent study, with higher HF rates with increased mean left ventricular dose (MLVD); relative to 0 Gy, HF rates following MVLD of 1–15, 16–20, 21–25, and ≥26 Gy were 1.27, 1.65, 3.84, and 4.39, respectively (ptrend < 0.001) [40].
The definition of HF is universally agreed as a clinical syndrome with a set of signs and symptoms of poor perfusion and cardiac output, secondary to structural or functional impairment in cardiac function [41]. These changes are further classified by ejection fraction (EF) as heart failure with preserved ejection fraction (HFpEF; EF≥50%), heart failure with mildly reduced ejection fraction (HFmrEF; EF: 41-49%), and heart failure with reduced ejection fraction (HFrEF; EF≤40%) [42]. Although HF in patients with prior RT could be multifactorial (constrictive pericarditis, hypertrophy from valvular disease, ischemic heart disease), it predominantly causes restrictive cardiomyopathy with diastolic dysfunction and fibrosis [18,19,41]. The most common echocardiographic findings were regional wall-motion abnormalities (often inferior in location), mild global LV hypokinesia, depressed LV systolic function, impaired myocardial relaxation, and diastolic dysfunction [6]. The right ventricle was more affected than the LV, likely secondary to its anterior location [19].
Diastolic dysfunction has been more widely reported and linked to RT than systolic dysfunction. In a study of survivors of Hodgkin's lymphoma who received >35 Gy mediastinal radiation, 14% had evidence of diastolic dysfunction on echocardiography [43]. In addition, a recent case-control study of females with HF who received RT for breast cancer, found that 64% of HF cases had HFpEF (EF >50%), and 89% had EF >40% [44]. This is likely explained by the underlying pathophysiologic process of RT-mediated myocardial toxicity. Since cardiomyocytes are non-proliferating cells, they are resistant to radiation effects. However, the rapidly proliferating endothelial cells are more prone, leading to microvascular endothelial damage, oxidative stress, myocardial inflammation, and fibrosis. Those microvascular changes are shared with co-morbidity-driven myocardial effects identified as the key factor in the pathophysiology of HFpEF, while cardiomyocyte death secondary to infarction or other factors is the major insult in HFrEF [45,46].
EF has been conventionally used as a surrogate and predictor of late myocardial radiotoxicity [6,7]. However, two significant issues accompanied this practice. First, the definition of cancer therapy-related cardiac dysfunction is not well-established, with different studies using variable arbitrary cutoffs and percentages of decline in EF. It may include an EF decline of >20% (EF units), a decrease of left ventricular ejection fraction (LVEF) by >10 points to <55%, or a drop of LVEF <45% [6,45]. Therefore, LVEF-based definitions lack reproducibility and do not address many patients with HFpEF. Second, LVEF can be insensitive in detecting early signs of radiotoxic myocardial damage. Hence, its value in predicting late cardiotoxicity is debatable [6]. Therefore, other methods have been proposed, such as two-dimensional speckle tracking echocardiography (2D-STE) [7]. A 2D-STE aims to analyze strain patterns in three different dimensions of contractility (longitudinal, circumferential, and radial) and measure strain rates (SR) during systole (SRs), early (SRe), and late (SRa) diastole. Its principal value is detecting systolic and diastolic myocardial dysfunction when EF remains normal [47,48].
Standard medical therapy is used similarly for HF symptoms arising from radiation, utilizing beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), and diuretics; however, data about the role of standard HF medical therapy among RT recipients are scarce. Heart transplant is an option for end-stage cardiac dysfunction with a lower post-transplant 5-year survival rate compared to other causes of cardiomyopathy, imposing a higher operative risk in radiated patients [19].
5. Coronary artery disease
CAD is one of the common manifestations of RIHD, presenting in the form of stable angina, acute coronary syndrome, ischemic cardiomyopathy, and HF [8]. Clinical studies have shown an estimated incidence of CAD of up to 85% [19]. A large population study showed that the rate of coronary events increases by 7.4% for every 1 Gy increase in MHD, with damage starting within 5 years and extending up to 20 years of exposure [49]. The damage is triggered by endothelial injury leading to oxidative stress and proinflammatory and prothrombotic states, which cause intimal thickening and accelerated atherosclerosis and stenosis [19,50]. The arterial narrowing is mainly seen in the ostial and proximal portions of epicardial vessels [19]. The distribution of coronary involvement depends on the chest site receiving radiation. For example, mediastinal radiation is associated with LAD and RCA involvement [18]. LBC radiation involves the middle and distal segments of LAD, while RBC radiation involves the proximal portion of the RCA [50].
Multiple factors influence the radiation-mediated damage to coronary vessels, including the side of radiation and the dose delivered. For instance, a study performed by Tagami et al. [51] showed that LBC patients treated with RT have a significantly higher incidence of coronary disease when compared with a matched group of patients treated for RBC. Amongst others, mean LAD radiation dose and MHD strongly correlated with coronary disease, with a 21% higher incidence of disease in the LAD per Gy when considering the mean LAD dose and a 95% higher incidence of disease in the LAD per Gy when considering the MHD. In addition, there is a linear dose-response relationship between mean radiation dose and risk of CAD. A study in Hodgkin's lymphoma patients showed a 2.5-fold increase in risk with mean doses of ≥20 Gy. However, recent evidence suggests that MHD may not accurately represent the magnitude of coronaries' exposure [50]. A study done by Jacob et al. [12] on LBC radiation recipients showed that despite a low MHD of <3 Gy, 56% of patients could nevertheless still be receiving LAD doses above 40 Gy. Furthermore, radiation doses to LAD atherosclerotic plaques have also been studied and found to be the strongest predictor of acute coronary events (ACEs) amongst patients with established atherosclerotic disease, even after correction for cardiovascular risk factors (hazard ratio [HR] = 1.269; 95% CI, 1.090–1.477; p = 0.002). However, the volume of the left ventricle receiving ≥5 Gy remains to be an essential predictor of ACEs in patients without atherosclerotic plaques in the LAD (n = 680) (HR = 1.021; 95% CI, 1.003–1.039; p = 0.023) [52].
Multiple modalities contribute to the diagnosis of radiation-related CAD. Echocardiography can be utilized to detect wall motion abnormalities at rest yet may not be sufficient to detect induced ischemia [7]. Stress echocardiography has been studied, and its particularly high sensitivity and specificity in detecting epicardial vessel abnormality makes it a reliable for identifying transient myocardial ischemia [6]. A study performed on lymphoma patients who received mediastinal radiation >35 Gy utilized stress echo for evaluating CAD and showed a prevalence of 7.5% 15 years after RT. The positive predictive values for stress echocardiography were 80% and 87% for detecting ≥70% and ≥50% coronary stenosis, respectively [53]. The value of coronary calcium scoring has also been mentioned in the literature. A multicenter cohort study evaluated the correlation between coronary artery calcium (CAC) and CAD. They found that CAC on breast cancer RT planning CT scan results was associated with CVD, especially CAD, particularly in CAC score >400 [54]. Treatment of radiation-induced CAD with common revascularization techniques is challenging, as those patients usually have more complex anatomy and disease complexity, scarred and fibrotic grafts and higher rates of postoperative complications, such as poor healing, infections and bleeding, and higher mortality as compared to CAD from other causes [18,19].
Risk Factors for RIHD
Several radiation-related and patient-related risk factors are implicated in RIHD [6,18] (Fig. 1).
Fig. 1.
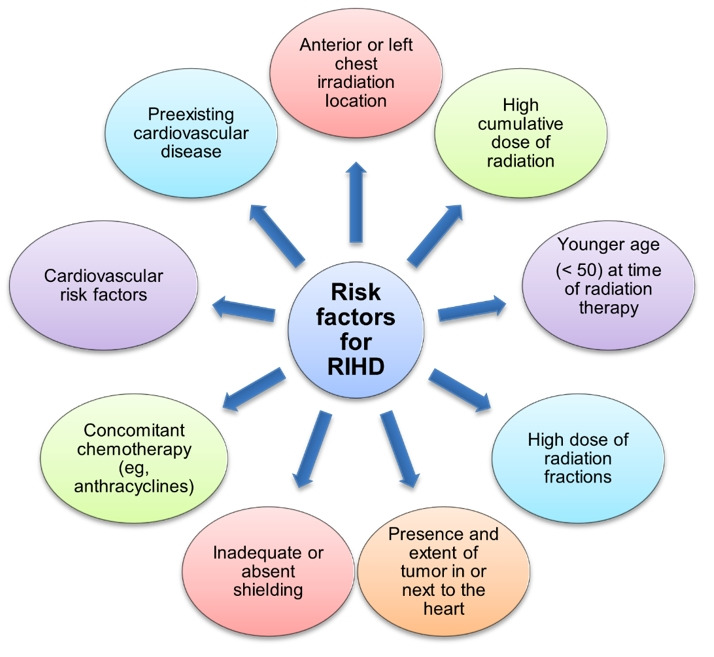
Schematic diagram illustrating the risk factors for radiation-induced heart disease (RIHD).
1. Radiation-related risk factors
The irradiated heart volume is the major risk factor for the development of RIHD [55]. Patients with breast cancer who had the highest doses and volumes of radiation to the heart had a threefold greater risk of cardiac death than those who underwent surgery alone after a mean follow-up of 16 years [4]. The same study has also shown that RT to left-sided tumors resulted in higher exposure to the heart than RT to right-sided tumors [4]. Radiation doses above 30 Gy reportedly increased RIHD by three and a half folds [18,56]. Some of the studies about radiation-related risk factors are summarized in Table 1 [47-64].
Table 1.
Summary of study findings related to radiation-related risk factors
| Study | Study findings |
|---|---|
| Chung et al. [57] | Significant MHD effect per gray for cardiac toxicity in Asian women with breast cancer. |
| The detrimental effect of radiation on the heart is independent of other cardiac risk factors. | |
| Atkins et al. [58] | Optimal cardiac dose constraints may differ based on preexisting coronary heart disease. |
| Left anterior descending coronary artery volume receiving 15 Gy greater than or equal to 10% is an independent estimator of the probability of major adverse cardiac events and all-cause mortality, particularly in patients without coronary heart disease. | |
| Left ventricle volume receiving 15 Gy greater than or equal to 1% is associated with an increased risk of major adverse cardiac events among patients with coronary heart disease. | |
| Jang et al. [59] | A high left ventricle radiation dose could raise adverse cardiovascular events in patients with stage III NSCLC and increased cardiovascular risk. |
| Pre-treatment evaluation of cardiac risk and individualized surveillance may help prevent cardiac events post-chemoradiotherapy. | |
| Morris et al. [60] | Deep learning poses extensive efficiency and accuracy gains for cardiac substructure segmentation, offering the increased potential for rapid implementation into radiation therapy planning for improved cardiac sparing. |
| Clasen et al. [61] | Modest subclinical changes in cardiac function measures were seen in the short term with use of modern radiation planning techniques. |
| Atkins et al. [62] | Cardiac radiation dose exposure is a cardiac risk factor for major adverse cardiac events and all-cause mortality in advanced NSCLC. |
| Early recognition of cardiovascular events along with their treatment and more stringent avoidance of increased cardiac radiotherapy dose is required. | |
| Boggard et al. [63] | A significant dose-effect relationship was found for acute coronary events within nine years after radiation therapy. |
| Left ventricle receiving 5 Gy seemed to be a better prognosticator for adverse cardiac events than MHD. | |
| Reducing the exposure of the heart to radiation is essential to avoid the excess risk of acute coronary events after radiotherapy for breast cancer. | |
| Taylor et al. [64] | For individual left ventricle and coronary artery segments, increased radiation doses were strongly associated with more frequent injury |
| All segments are sensitive to radiation, and doses to all segments should be minimized. |
MHD, mean heart dose; NSCLC, non-small cell lung cancer.
2. Patient-related risk factors
Multiple patient-related factors have been found to alter the risk of RIHD. Studies have shown that younger patients have an increased risk of RIHD [65]. Van Nimwegen et al. [66] demonstrated that patients younger than 25 years old, when treated for Hodgkin's lymphoma, had a 4.6 to 7.6-fold higher risk of radiation-induced CAD. Classic cardiovascular risk factors such as diabetes, hypertension, dyslipidemia, family history of CAD, as well as established cardiovascular disease has also been linked to a higher risk of RIHD [18,19]. This was also demonstrated in a recent study of breast cancer in the Korean population, which showed that the risk of ACEs and cardiac mortality were lower in healthy women. Obesity was an independent risk factor for the development of cardiac mortality, with an increased risk of 5% in cardiac death for each increment of 1 kg/m2 in body mass index (BMI) [67]. The study also demonstrated that the 10-year mortality rate owing to the non-cancer origin was only 1%–2% amongst Korean breast cancer survivors, significantly different from their North American counterparts, likely attributable to the general characteristics of the Korean breast cancer population, who generally smoke less, weigh less, and have fewer known risk factors for heart disease [67]. In addition, smoking, when added to RT, conferred a three times higher hazard for myocardial infarction [68]. In terms of physical activity, a study on Korean breast cancer survivors compared to the general Korean population showed no significant difference in the risk of ACEs. However, in the sensitivity and subgroup analyses, breast cancer survivors had increased risks of ACEs if they did not exercise or had a disability [69]. Concurrent use of chemotherapeutic agents, particularly anthracyclines, also further increases the likelihood of cardiac events [6,19].
Role of Biomarkers
Several biomarkers, including troponin T (TnT), troponin I (TnI), and brain natriuretic peptide (BNP), are indicators of cardiac injury that have roles in the evaluation of patients post-RT [5]. High sensitivity TnT (hsTnT) is an indicator of myocardial damage. High sensitivity TnT was elevated in 21% of breast cancer patients during RT, particularly those who received higher RT doses for the whole heart and LV [70]. BNP has the potential to serve as a marker for predicting cardiac events after RT [71]. The BNP levels were elevated immediately post-RT in patients with thoracic malignancies receiving chemoradiation; however, it rose over time in the patients who received RT only [72]. Overexpression of insulin-like growth factor 1 receptor β correlated with a decrease in overall survival, and therefore it has the potential to be considered a biomarker of radiation resistance [73].
Xu et al. [74], in their study, stated that elevation of hsTnT during chemoradiation therapy was radiation heart dose-dependent and was associated with cardiac adverse events and mortality. They further suggested that routine monitoring of hsTnT could help identify patients who are at high risk of chemoradiation-induced cardiac adverse events at an earlier stage to guide modifications of cancer treatment and possible interventions, thereby mitigating cardiotoxicity.
Screening and Surveillance after Chest Radiation Therapy: The Expert Consensus
Little is known about the overall prevalence of pre-clinical RIHD and whether early screening would benefit asymptomatic patients. However, due to the disease burden, close follow-up and monitoring for signs and symptoms are warranted by the current guidelines. Consensus guidelines from the EACVI and ASE, published in 2013, propose a screening and follow-up algorithm for patients receiving chest radiation [6]. Those are the most established surveillance guidelines up to date. In addition, The American and European Society of Medical Oncology have also issued guidelines for the prevention, diagnosis, and management of cardiovascular disease associated with cancer therapy [75].
All guidelines recommend aggressive screening and management of cardiovascular risk factors in patients receiving chest radiation; this includes initial identification of risk factors, a comprehensive clinical exam, and a baseline transthoracic echocardiography. Following the initiation of RT, patients are to be monitored yearly with history and clinical exams. Any development of new symptoms (dyspnea, paroxysmal nocturnal dyspnea, orthopnea, angina, syncope, palpitations) or exam findings such as new murmur warrants further investigation using echocardiography, CT coronary, or CMR. Asymptomatic patients are screened based on their baseline risk factors (Fig. 2). Those who have no risk factors are screened with transthoracic echocardiography ten years after RT exposure. If no abnormalities are detected, reassessment occurs every 5 years. However, patients who possess a set of risk factors receive screening echocardiography 5 years post-exposure. In addition, due to the increased risk of coronary involvement in this population, non-invasive stress testing is recommended at 5 years. Stress echocardiography and stress MRI have higher specificity than stress ECG and are generally preferred. Revaluation can happen at a 5-year interval if the initial assessment is normal. Fig. 2 provides an algorithmic representation of the above recommendations. The role of cardiac CT and CMR in screening is yet to be identified. As of now, they serve as complementary imaging modalities for further identification of abnormalities detected on echocardiography or for preoperative cardiac evaluation [6,19].
Fig. 2.
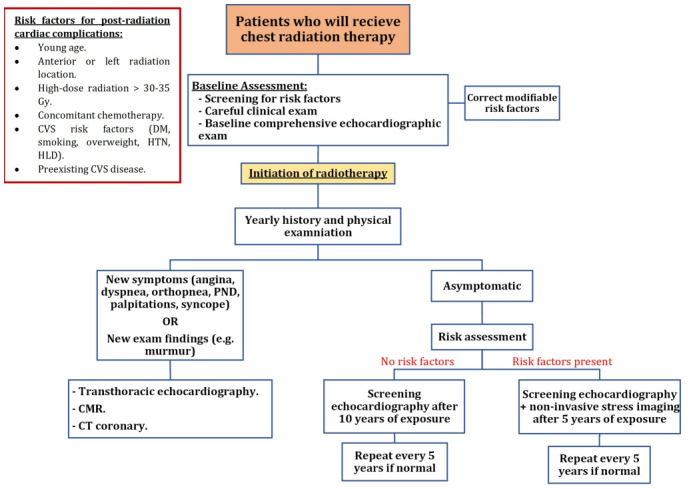
Algorithmic demonstrations of screening guidelines of radiation-induced heart disease (RIHD). Adapted from the European Association of Cardiovascular Imaging (EACVI) and American Society of Echocardiography (ASE) guidelines, 2013. CMR, cardiac magnetic resonance; CT, computed tomography; CVS, cardiovascular system; DM, diabetes mellitus; HLD, hyperlipidemia; HTN, hypertension; PND, paroxysmal nocturnal dyspnea.
Extensive prospective studies are further required to understand the mechanisms behind RIHD further and confirm the clinical utility of non-invasive imaging and dose-volume analysis in predicting the risk and magnitude of the disease. The RACCOON (Radiotherapy for Thoracic and Breast Cancer and the Related Cardiotoxicity Following Treatment; ClinicalTrials.gov Identifier: NCT04674501) trial is a promising ongoing Korean prospective observational study that further explores the above targets in a wide range of malignancy patients who will receive chest RT, to report outcomes of cardiotoxicity rate, overall survival, and cancer-specific survival rates.
Diagnostic Approach and Management
The diagnostic approach to RIHD depends on the presenting signs and symptoms and suspected disease. A brief description of diagnostic modalities was mentioned above in corresponding sections of different cardiac manifestations. Clinicians will have to use available techniques of echocardiography, CT, CMR, or SPECT scan for the appropriate clinical indication. Echocardiography is a non-invasive first-line imaging modality for most cardiac manifestations, including pericardial disease, myocardial dysfunction, and VHD. Echocardiography is helpful in detecting and serial monitoring pericardial effusion and constrictive pericarditis, yet CT and CMR are more specific in detecting certain anatomical abnormalities such as thickenings and calcifications. In addition, echocardiography provides an assessment of LV systolic and diastolic function, and 2D speckle tracking assesses strain patterns, all of which reflect myocardial function. CMR can provide an advanced evaluation in cases of a poor echocardiographic acoustic window and for detecting myocardial fibrosis. For coronary artery disease, echocardiography can assess the presence of wall motion abnormalities, yet image-based stress testing has a higher specificity for detecting anomalies, followed by appropriate CT coronary if needed [6,7,76].
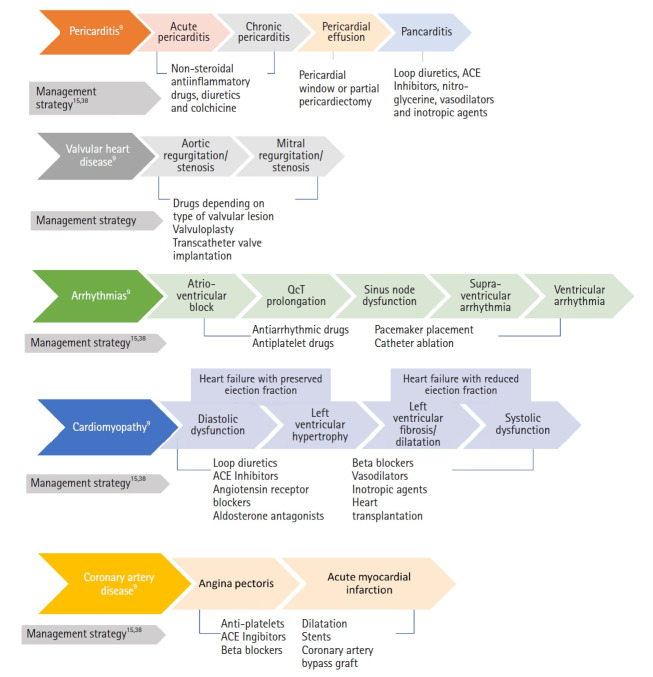
In terms of management, the current approach follows the same treatment options for non-RIHD, whether medical or surgical. The clinical spectrum and recommended management strategies of RIHD are described in Fig. 3. A recent review of the American and European Cardio-Oncology guidelines has proposed protocols and management algorithms for chemotherapy-induced cardiac toxicity depending on the developed complications. These include protocols for hypertension, systolic dysfunction and HF, atrial fibrillation, QT prolongation, and myocarditis [75]. Those protocols incorporate imaging modalities and biomarkers with suggested follow-up intervals and target cutoff for action. Those algorithms may serve as a guide for radiation-induced heart toxicity under the umbrella of cancer therapy-induced cardiac damage. As a general summary of surgical management outcomes, transcatheter aortic valve replacement has been shown to have less than 30 days of mortality compared to surgical aortic valve replacement valvular disease, coronary artery bypass graft surgery, and percutaneous coronary intervention had similar overall revascularization outcomes. Moreover, pericardiectomy has low 5-year survival rates. In general, if surgery is planned, an assessment of calcifications of thoracic vessels (thoracic aorta, internal mammary artery [IMA]), pulmonary function, and other cardiac comorbidities is required [19].
Fig. 3.
The clinical spectrum and recommended management strategies of radiation-induced heart diseases. ACE, angiotensin converting enzyme.
Preventive Pharmacologic Therapies in RIHD
Currently, there is no absolute modality to prevent radiation over-exposure to the heart primarily; however, advancements in RT technologies have lowered exposure of normal healthy tissue near the targeted tumor in the past few years.
Various technologies have been applied in clinical practice to reduce radiation dose and volume received by the heart, including deep inspiratory breath-hold (DIBH), three-dimensional conformal RT, intensity-modulated RT, 4D-CT, partial breast irradiation, and volumetric arc therapy [8,77-79]. Continuous positive airway pressure, a novel method to avoid heat exposure, has resulted in reasonably low irradiation to the heart [80].
In addition, high-dose radiation exposure to the LV can be precluded by a variety of heart-sparing radiation techniques like Cerrobend blocking or multileaf collimator (MLC), breath-hold, prone positioning, or deep inspiration [81]. With the help of modern RT technologies and a lower fraction of radiation therapy, the incidence of acute pericarditis and myocarditis has become less [18].
Furthermore, numerous radioprotectors that rely on selective uptake by normal organ tissues have been developed, with short and long-acting protectors successfully being utilized in modern practice [82]. Follow-up visits by patients and specific drug treatments are considered secondary preventative measures.
Investigational studies have documented that RIHD can be prevented by certain drugs, including statins, ACEIs, and anti-oxidants [83-85] but are in experimental stages.
1. Statins
Statins exert their anti-inflammatory effects by reducing oxidative stress and activating adenosine 5'-monophosphate-activate protein kinase (AMPK) [86]. Cardioprotection can be attained by inhibiting inflammatory reactions and oxidative stress. Zhang et al. [83] showed that atorvastatin could decrease radiation-induced myocardial fibrosis by preventing multiple inflammatory responses and oxidative stress pathways activation. Lovastatin has protective effects in both the acute and chronic phases through inhibition of NF-κB activation, ICAM expression, and miRNA expression of the CTGF [85].
Atkins et al. assessed the correlation between statin therapy and all-cause mortality, overall survival, and major adverse cardiac events (MACE) among patients with locally advanced NSCLC who received RT. They found that half of the statin-eligible high cardiac risk patients only were on statin therapy. In addition, those who were on statins harbored more significant risk factors than those not on a statin. Therefore, the former group had higher all-cause mortality despite statin therapy; however, similar rates of MACE. Furthermore, they found that statin naïve patients who received radiation doses >10 Gy had higher all-cause mortality than <10 Gy doses. This finding was not valid in patients on statin therapy, suggesting the need for further studies to mitigate the effect of statin on high cardiac risk patients who receive high radiation doses [87].
2. Angiotensin-converting enzyme inhibitors
ACEIs also reduce reactive oxygen species production and decrease oxidative stress and inflammatory mediated cardiac injury. ACEIs drugs can attenuate myocardial perivascular fibrosis and myocardial cell apoptosis through these protective mechanisms, thus preventing myocardial fibrosis and reducing cardiac diastolic dysfunction [84]. Van der Veen et al. [84] demonstrated the protective effects of captopril against RIHD. Captopril was associated with improvements in the breathing rate and cardiopulmonary density and reduced pleural and pericardial effusion and cardiac fibrosis.
3. Anti-inflammatory and antioxidants
The acute phase inflammatory response and oxidative stress play significant roles in developing RIHD; thus, anti-inflammatory and antioxidant drugs can have substantial cardioprotective effects. Colchicine, for example, has anti-inflammatory properties, which could be attributed to its inhibition of microtubule polymerization resulting in reduced platelet aggregation and its reduced expression of endothelial and leukocyte adhesion molecules. Hence colchicine has been proposed to protect from radiation-induced CAD through its anti-inflammatory and anti-coagulant properties [88]. A pre-clinical trial on caffeic acid phenethyl ester (CAPE) revealed its suppressive effect on the acute inflammatory response. CAPE-induced anti-oxidant properties were able to reduce gamma radiation-induced myocardial injury [89]. Another study by Boerma et al. [90] revealed that pentoxifylline could reduce endothelial dysfunction and prevent the downregulation of endothelial cell surface thrombomodulin. In combination with alpha-tocopherol, pentoxifylline showed a significant reduction in collagen I and III deposition, which was thought to occur by inhibiting intracellular TGF-β and CTGF expression and reducing LV diastolic pressure. Furthermore, amifostine was found to have protection against RT-induced cardiac fibrosis, myocardial degeneration, and vascular damage [91-93].
Small molecule TGF-βR1 inhibitor, PW-5371, was found to reduce cardiopulmonary fibrosis and prevent chronic radiation-induced cardiopulmonary dysfunction in mice [94]. Recombinant human neuregulin-1 (rhNRG-1β) has continuous protective properties against myocardial damage and preserves cardiac function by activating the ErbB2-ERK-SIRT1 signaling transduction pathway [95]. Natural products including melatonin, hesperidin, and curcumin have also been shown to decrease radiation-induced cardiac injury through their anti-oxidant and anti-inflammatory properties [96].
Conclusion
The increasing use of RT for cancer treatment may give rise to a greater risk of RIHD if suitable preventative and screening procedures are not in place. RIHD represents a range of cardiac pathology, including CAD, cardiomyopathy, pericardial disease, valvular disease, and conduction abnormalities. Certain drugs may help mitigate the effects of RT and treat RIHD; however, their clinical application/use as radioprotectors and specific treatments to halt the progression of RIHD is far-fetched. Global implementation of guidelines and advances in RT can help in earlier detection and less frequent occurrence of RIHD. Ongoing research in clinical and lab biomarkers may assist in the risk stratification of patients at a higher risk of developing cardiac complications in the future.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization, SE, YM, ME. Supervision, SE, YM. Writing of the original draft, SE, AK. Writing of the review and editing, SE, AK, YM, ME, ND. Validation, AK, YM, ME, ND, SE. All the authors have proofread the final version.
Data Availability Statement
Not applicable.
References
- 1.Slezak J, Kura B, Babal P, et al. Potential markers and metabolic processes involved in the mechanism of radiation-induced heart injury. Can J Physiol Pharmacol. 2017;95:1190–203. doi: 10.1139/cjpp-2017-0121. [DOI] [PubMed] [Google Scholar]
- 2.Donnellan E, Phelan D, McCarthy CP, Collier P, Desai M, Griffin B. Radiation-induced heart disease: a practical guide to diagnosis and management. Cleve Clin J Med. 2016;83:914–22. doi: 10.3949/ccjm.83a.15104. [DOI] [PubMed] [Google Scholar]
- 3.Om A, Ellahham S, Vetrovec GW. Radiation-induced coronary artery disease. Am Heart J. 1992;124:1598–602. doi: 10.1016/0002-8703(92)90078-a. [DOI] [PubMed] [Google Scholar]
- 4.Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–96. doi: 10.1016/0360-3016(92)90784-f. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf SW, Venkatesulu BP, Mahadevan LS, Krishnan S. Radiation-induced cardiovascular disease: a clinical perspective. Front Cardiovasc Med. 2017;4:66. doi: 10.3389/fcvm.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–40. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 7.Quintero-Martinez JA, Cordova-Madera SN, Villarraga HR. Radiation-induced heart disease. J Clin Med. 2021;11:146. doi: 10.3390/jcm11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Wei J, Zheng Q, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15:2128–38. doi: 10.7150/ijbs.35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L, Lei D, Wang W, Luo Y, Wang D. Heart dose linked with cardiac events and overall survival in lung cancer radiotherapy: a meta-analysis. Medicine (Baltimore) 2020;99:e21964. doi: 10.1097/MD.0000000000021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis M, Witteles RM. Radiation-induced heart disease: an under-recognized entity? Curr Treat Options Cardiovasc Med. 2014;16:317. doi: 10.1007/s11936-014-0317-2. [DOI] [PubMed] [Google Scholar]
- 11.Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–7. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Jacob S, Camilleri J, Derreumaux S, et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study) Radiat Oncol. 2019;14:29. doi: 10.1186/s13014-019-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivekanandan S, Landau DB, Counsell N, et al. The impact of cardiac radiation dosimetry on survival after radiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99:51–60. doi: 10.1016/j.ijrobp.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkozy M, Varga Z, Gaspar R, et al. Pathomechanisms and therapeutic opportunities in radiation-induced heart disease: from bench to bedside. Clin Res Cardiol. 2021;110:507–31. doi: 10.1007/s00392-021-01809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boero IJ, Paravati AJ, Triplett DP, et al. Modern radiation therapy and cardiac outcomes in breast cancer. Int J Radiat Oncol Biol Phys. 2016;94:700–8. doi: 10.1016/j.ijrobp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Boekel NB, Schaapveld M, Gietema JA, et al. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys. 2016;94:1061–72. doi: 10.1016/j.ijrobp.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract. 2011;2011:317659. doi: 10.4061/2011/317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutroumpakis E, Palaskas NL, Lin SH, et al. Modern radiotherapy and risk of cardiotoxicity. Chemotherapy. 2020;65:65–76. doi: 10.1159/000510573. [DOI] [PubMed] [Google Scholar]
- 19.Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc. 2021;10:e021686. doi: 10.1161/JAHA.121.021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 21.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–73. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 22.McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–75. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–8. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 24.Niedzielski JS, Wei X, Xu T, et al. Development and application of an elastic net logistic regression model to investigate the impact of cardiac substructure dose on radiation-induced pericardial effusion in patients with NSCLC. Acta Oncol. 2020;59:1193–200. doi: 10.1080/0284186X.2020.1794034. [DOI] [PubMed] [Google Scholar]
- 25.Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart. 2016;102:269–76. doi: 10.1136/heartjnl-2015-308765. [DOI] [PubMed] [Google Scholar]
- 26.Zafar MR, Mustafa SF, Miller TW, Alkhawlani T, Sharma UC. Outcomes after transcatheter aortic valve replacement in cancer survivors with prior chest radiation therapy: a systematic review and meta-analysis. Cardiooncology. 2020;6:8. doi: 10.1186/s40959-020-00062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107:djv008. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wethal T, Lund MB, Edvardsen T, et al. Valvular dysfunction and left ventricular changes in Hodgkin's lymphoma survivors: a longitudinal study. Br J Cancer. 2009;101:575–81. doi: 10.1038/sj.bjc.6605191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cella L, Liuzzi R, Conson M, et al. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkin's lymphoma. Radiother Oncol. 2011;101:316–21. doi: 10.1016/j.radonc.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 30.Cella L, D'Avino V, Liuzzi R, et al. Multivariate normal tissue complication probability modeling of gastrointestinal toxicity after external beam radiotherapy for localized prostate cancer. Radiat Oncol. 2013;8:221. doi: 10.1186/1748-717X-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–85. doi: 10.1161/CIRCULATIONAHA.113.001435. [DOI] [PubMed] [Google Scholar]
- 32.Bouleti C, Amsallem M, Touati A, et al. Early and late outcomes after trans-catheter aortic valve implantation in patients with previous chest radiation. Heart. 2016;102:1044–51. doi: 10.1136/heartjnl-2015-309101. [DOI] [PubMed] [Google Scholar]
- 33.Donnellan E, Masri A, Johnston DR, et al. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6:e005396. doi: 10.1161/JAHA.116.005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–28. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 35.Qian Y, Zhu H, Pollom EL, et al. Sinoatrial node toxicity after stereotactic ablative radiation therapy to lung tumors. Pract Radiat Oncol. 2017;7:e525. doi: 10.1016/j.prro.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Trapani G, Quartuccio S, Dalbeni A, Stellitano A, Paunovic N, Imbalzano E. Late radiation-induced cardiac conduction system abnormalities. Int J Cardiol. 2014;173:e40–1. doi: 10.1016/j.ijcard.2014.03.125. [DOI] [PubMed] [Google Scholar]
- 37.Nakao T, Kanaya H, Namura M, et al. Complete atrioventricular block following radiation therapy for malignant thymoma. Jpn J Med. 1990;29:104–10. doi: 10.2169/internalmedicine1962.29.104. [DOI] [PubMed] [Google Scholar]
- 38.Bates J, Shrestha S, Liu Q, et al. OC-0208 Cardiac substructure dosimetry and late cardiac arrhythmia in the Childhood Cancer Survivor Study. Radiot Oncol. 2021;161(Suppl 1):S140–1. [Google Scholar]
- 39.Finch W, Shamsa K, Lee MS. Cardiovascular complications of radiation exposure. Rev Cardiovasc Med. 2014;15:232–44. doi: 10.3909/ricm0689. [DOI] [PubMed] [Google Scholar]
- 40.van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129:2257–65. doi: 10.1182/blood-2016-09-740332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Buono MG, Buckley L, Abbate A. Primary and secondary diastolic dysfunction in heart failure with preserved ejection fraction. Am J Cardiol. 2018;122:1578–87. doi: 10.1016/j.amjcard.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 43.Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977–82. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Saiki H, Petersen IA, Scott CG, et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–96. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloom MW, Hamo CE, Cardinale D, et al. Cancer therapy-related cardiac dysfunction and heart failure. Part 1: Definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9:e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 47.Cordova-Madera S, Garcia-Bello L, Bruno G, et al. Comprehensive strain analysis in oncological patients undergoing thoracic radiotherapy: 1-year follow-up of a prospective study comparing proton vs. photon beam therapy. Eur Heart J. 2021;42(Suppl_1):ehab724.024 [Google Scholar]
- 48.Sritharan HP, Delaney GP, Lo Q, Batumalai V, Xuan W, Thomas L. Evaluation of traditional and novel echocardiographic methods of cardiac diastolic dysfunction post radiotherapy in breast cancer. Int J Cardiol. 2017;243:204–8. doi: 10.1016/j.ijcard.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 50.da Silva RMFL. Effects of radiotherapy in coronary artery disease. Curr Atheroscler Rep. 2019;21:50. doi: 10.1007/s11883-019-0810-x. [DOI] [PubMed] [Google Scholar]
- 51.Tagami T, Almahariq MF, Balanescu DV, et al. Usefulness of coronary computed tomographic angiography to evaluate coronary artery disease in radiotherapy-treated breast cancer survivors. Am J Cardiol. 2021;143:14–20. doi: 10.1016/j.amjcard.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 52.van den Bogaard VA, Spoor DS, van der Schaaf A, et al. The importance of radiation dose to the atherosclerotic plaque in the left anterior descending coronary artery for radiation-induced cardiac toxicity of breast cancer patients? Int J Radiat Oncol Biol Phys. 2021;110:1350–9. doi: 10.1016/j.ijrobp.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25:43–9. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 54.Gal R, van Velzen SGM, Hooning MJ, et al. Identification of risk of cardiovascular disease by automatic quantification of coronary artery calcifications on radiotherapy planning CT scans in patients with breast cancer. JAMA Oncol. 2021;7:1024–32. doi: 10.1001/jamaoncol.2021.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S77–85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 56.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–55. [PubMed] [Google Scholar]
- 57.Chung SY, Oh J, Chang JS, et al. Risk of cardiac disease in patients with breast cancer: impact of patient-specific factors and individual heart dose from three-dimensional radiation therapy planning. Int J Radiat Oncol Biol Phys. 2021;110:473–81. doi: 10.1016/j.ijrobp.2020.12.053. [DOI] [PubMed] [Google Scholar]
- 58.Atkins KM, Chaunzwa TL, Lamba N, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. 2021;7:206–19. doi: 10.1001/jamaoncol.2020.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang BS, Cha MJ, Kim HJ, et al. Heart substructural dosimetric parameters and risk of cardiac events after definitive chemoradiotherapy for stage III non-small cell lung cancer. Radiother Oncol. 2020;152:126–32. doi: 10.1016/j.radonc.2020.09.050. [DOI] [PubMed] [Google Scholar]
- 60.Morris ED, Ghanem AI, Dong M, Pantelic MV, Walker EM, Glide-Hurst CK. Cardiac substructure segmentation with deep learning for improved cardiac sparing. Med Phys. 2020;47:576–86. doi: 10.1002/mp.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clasen SC, Shou H, Freedman G, et al. Early cardiac effects of contemporary radiation therapy in patients with breast cancer. Int J Radiat Oncol Biol Phys. 2021;109:1301–10. doi: 10.1016/j.ijrobp.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73:2976–87. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 63.van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35:1171–8. doi: 10.1200/JCO.2016.69.8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor C, McGale P, Bronnum D, et al. Cardiac structure injury after radiotherapy for breast cancer: cross-sectional study with individual patient data. J Clin Oncol. 2018;36:2288–96. doi: 10.1200/JCO.2017.77.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–8. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 66.van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–17. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 67.Chang JS, Ko BK, Bae JW, et al. Radiation-related heart disease after breast cancer radiation therapy in Korean women. Breast Cancer Res Treat. 2017;166:249–57. doi: 10.1007/s10549-017-4398-y. [DOI] [PubMed] [Google Scholar]
- 68.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–75. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 69.Chang JS, Shin J, Park EC, Kim YB. Risk of cardiac disease after adjuvant radiation therapy among breast cancer survivors. Breast. 2019;43:48–54. doi: 10.1016/j.breast.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Skytta T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:141. doi: 10.1186/s13014-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palumbo I, Palumbo B, Fravolini ML, et al. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: a prospective study. Breast. 2016;25:45–50. doi: 10.1016/j.breast.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Gomez DR, Yusuf SW, Munsell et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J Thorac Oncol. 2014;9:1554–60. doi: 10.1097/JTO.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreno-Acosta P, Vallard A, Carrillo S, et al. Biomarkers of resistance to radiation therapy: a prospective study in cervical carcinoma. Radiat Oncol. 2017;12:120. doi: 10.1186/s13014-017-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu T, Meng QH, Gilchrist SC, et al. Assessment of prognostic value of high-sensitivity cardiac troponin t for early prediction of chemoradiation therapy-induced cardiotoxicity in patients with non-small cell lung cancer: a secondary analysis of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 2021;111:907–16. doi: 10.1016/j.ijrobp.2021.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexandre J, Cautela J, Ederhy S, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J Am Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35:612–23. doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trifiletti DM, Wijesooriya K, Moyer G, et al. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy during deep inspiratory breath hold for left-sided whole-breast irradiation: a comparative analysis. J Radiother Pract. 2016;15:99–106. [Google Scholar]
- 78.Beck RE, Kim L, Yue NJ, Haffty BG, Khan AJ, Goyal S. Treatment techniques to reduce cardiac irradiation for breast cancer patients treated with breast-conserving surgery and radiation therapy: a review. Front Oncol. 2014;4:327. doi: 10.3389/fonc.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Sherif O, Yu E, Xhaferllari I, Gaede S. Assessment of intrafraction breathing motion on left anterior descending artery dose during left-sided breast radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1075–82. doi: 10.1016/j.ijrobp.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 80.Choi MS, Chang JS, Park RH, et al. Heart-sparing capability and positional reproducibility of continuous positive airway pressure in left-sided breast radiation therapy. doi: 10.1016/j.prro.2021.12.016. Pract Radiat Oncol 2022:S1879-8500(22)00050-9. [DOI] [PubMed] [Google Scholar]
- 81.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aliper AM, Bozdaganyan ME, Sarkisova VA, et al. Radioprotectors.org: an open database of known and predicted radioprotectors. Aging (Albany NY) 2020;12:15741–55. doi: 10.18632/aging.103815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang K, He X, Zhou Y, et al. Atorvastatin ameliorates radiation-induced cardiac fibrosis in rats. Radiat Res. 2015;184:611–20. doi: 10.1667/RR14075.1. [DOI] [PubMed] [Google Scholar]
- 84.van der Veen SJ, Ghobadi G, de Boer RA, et al. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 85.Ostrau C, Hulsenbeck J, Herzog M, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92:492–9. doi: 10.1016/j.radonc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 86.Sun W, Lee TS, Zhu M, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–62. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 87.Atkins KM, Bitterman DS, Chaunzwa TL, et al. Statin use, heart radiation dose, and survival in locally advanced lung cancer. Pract Radiat Oncol. 2021;11:e459–67. doi: 10.1016/j.prro.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 88.O'Herron T, Lafferty J. Prophylactic use of colchicine in preventing radiation induced coronary artery disease. Med Hypotheses. 2018;111:58–60. doi: 10.1016/j.mehy.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 89.Mansour HH, Tawfik SS. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur J Pharmacol. 2012;692:46–51. doi: 10.1016/j.ejphar.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 90.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72:170–7. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tokatli F, Uzal C, Doganay L, et al. The potential cardioprotective effects of amifostine in irradiated rats. Int J Radiat Oncol Biol Phys. 2004;58:1228–34. doi: 10.1016/j.ijrobp.2003.09.071. [DOI] [PubMed] [Google Scholar]
- 92.Gurses I, Ozeren M, Serin M, Yucel N, Erkal HS. Histopathological efficiency of amifostine in radiation‑induced heart disease in rats. Bratisl Lek Listy. 2018;119:54–9. doi: 10.4149/BLL_2018_011. [DOI] [PubMed] [Google Scholar]
- 93.Kruse JJ, Strootman EG, Wondergem J. Effects of amifostine on radiation-induced cardiac damage. Acta Oncol. 2003;42:4–9. doi: 10.1080/0891060310002168. [DOI] [PubMed] [Google Scholar]
- 94.Rabender C, Mezzaroma E, Mauro AG, et al. IPW-5371 proves effective as a radiation countermeasure by mitigating radiation-induced late effects. Radiat Res. 2016;186:478–88. doi: 10.1667/RR14403.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu A, Jie Y, Sun L, Zhao S, E M, You Q. RhNRG-1β protects the myocardium against irradiation-induced damage via the ErbB2-ERK-SIRT1 signaling pathway. PLoS One. 2015;10:e0137337. doi: 10.1371/journal.pone.0137337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Musa AE, Shabeeb D. Radiation-induced heart diseases: protective effects of natural products. Medicina (Kaunas) 2019;55:126. doi: 10.3390/medicina55050126. [DOI] [PMC free article] [PubMed] [Google Scholar]