Fig. 1: Overview of next-generation CAR T cell approaches.
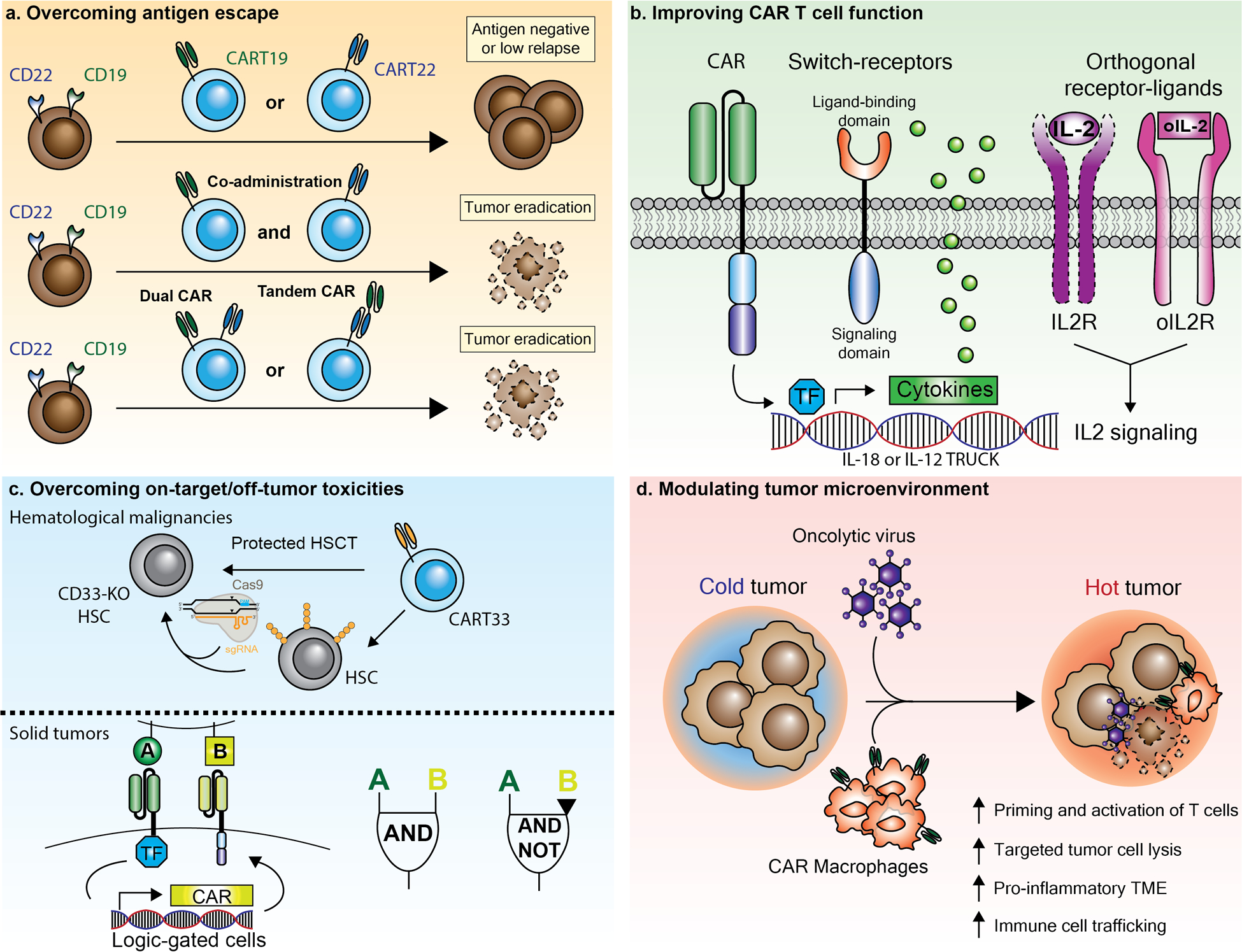
a) To reduce the risk of tumor relapse due to antigen-escape (e.g., loss or downregulation of CD19 or CD22), two CAR T cell products specific for different target antigens can be co-administered. The same T cell can also be transduced with two viral CAR vectors encoding for different constructs, or with one CAR construct with two single chain variable fragments (Dual or Tandem-CAR). b) CAR constructs with advanced functionalities include T cells redirected for antigen-unrestricted cytokine-initiated killing (TRUCK), which secrete cytokines upon activation (e.g., IL-18 or IL-12). In addition, CAR T cell function can be further enhanced by using switch-receptors (e.g., TGFβ), or through addition of orthogonal cytokine receptor system that act specifically on CAR-transduced cells (e.g., orthogonal IL2 receptor, oIL2R). TF= transcription factor. c) To overcome on-target / off-tumor toxicities in hematological malignancies, CAR T cells can be combined with hematopoietic stem cell transplantation (HSCT) that has been engineered to lack the CAR T cell target antigen (e.g., CD33 knock-out hematopoietic stem cells, CD33 KO HSC). For solid tumors, AND and NOT logic-gated CAR T cells have been developed that recognize combinatorial antigen expression to trigger or inhibit CAR T cell effector function. d) The additional administration of engineered viruses can effectively modulate the tumor microenvironment and engage innate / adaptive immunity. Also, CAR-macrophages can phagocytose tumor cells and induce anti-tumor bystander immune cells.
