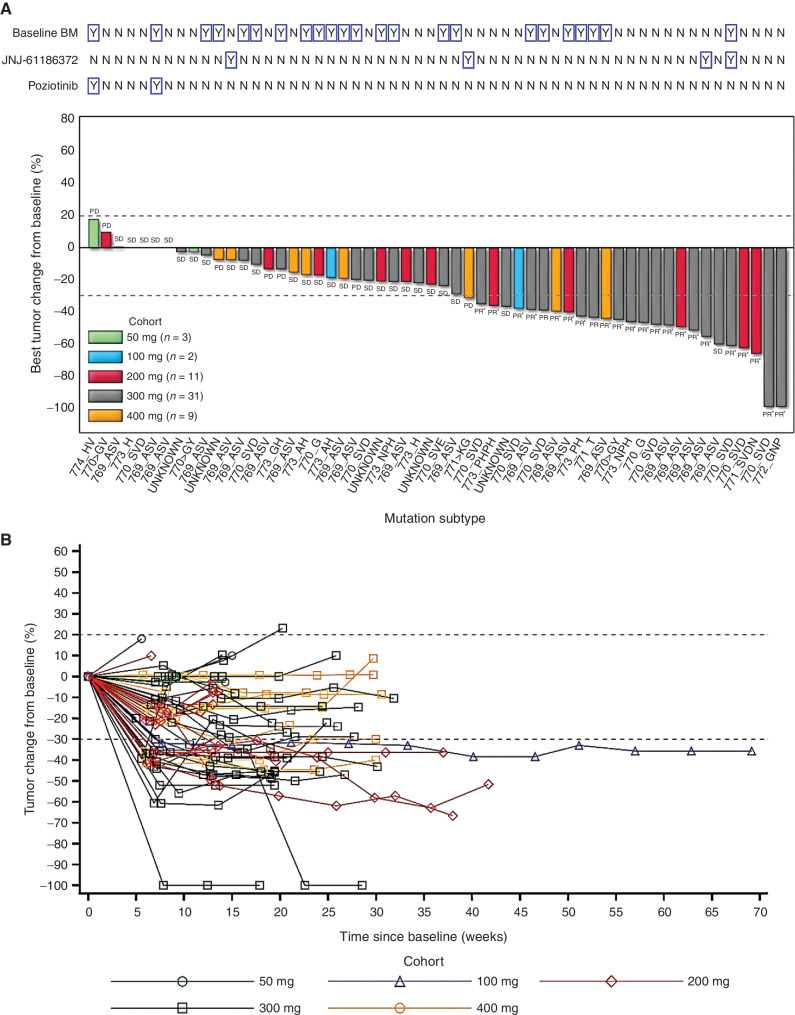
Figure 4.
Clinical activity of sunvozertinib in EGFRexon20ins NSCLC patients with postbaseline target lesion assessments. A, Best percentage change from baseline in target lesions by dose level, molecular subtype, prior amivantamab or poziotinib treatment status, and baseline BM. B, Plot showing percentage change from baseline in target lesion by time on treatment and dose level. Pooled analysis of WU-KONG1 and WU-KONG2 studies was performed. Data cutoff date: April 3, 2021. Tumor response was assessed by investigators according to RECIST 1.1. PD, progressive disease; SD, stable disease. *, confirmed response. EGFRexon20ins subtypes were confirmed by next-generation sequencing using tumor tissue or/and plasma circulating tumor DNA. JNJ-61186372 = amivantamab. Note: Among the 56 patients, a total of 45 subjects had tumor tissue and/or plasma samples tested by a central laboratory using next-generation of sequencing; 41 of 45 were confirmed as EGFRexon20ins-positive, and the overall concordance rate was 91%. However, 31 subjects had only tumor tissue tested by the central laboratory, and the concordance rate between local and central laboratory testing was 97% (30/31).