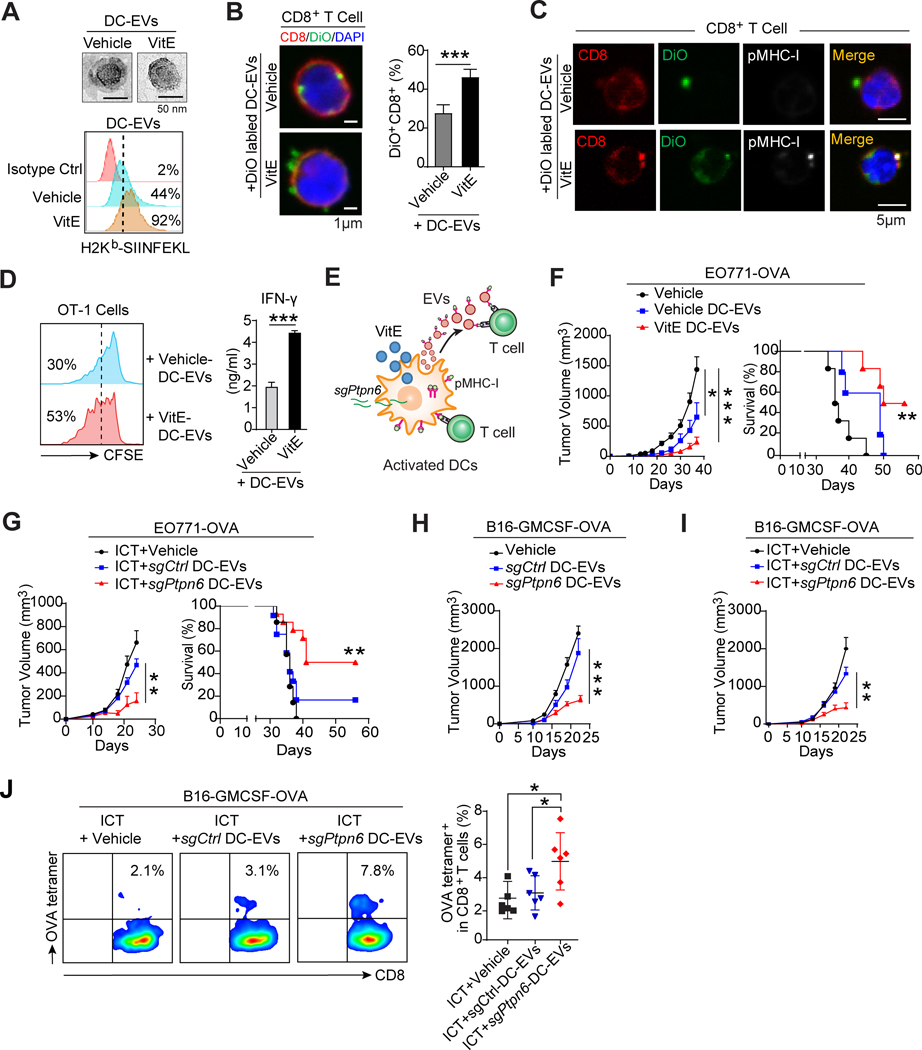
Figure 6. SHP1 inhibition enhances the antigen cross-presentation of DC-derived EVs to boost system antitumor immunity.
A, A representative transmission electron microscope (TEM) image of DC-EVs (top) and flow cytometric analysis of H-2Kb-SIINFEKL in DC-EVs (bottom) derived from OVA-loaded BMDCs treated with vehicle or VitE.
B, Confocal microscopy analysis (left) and quantification (right, n=5) of DiO+CD8+ T cells after T cells were incubated with vehicle- or VitE-treated DiO-labelled DC-EVs for 48 hrs.
C, Confocal microscopy analysis of pMHC-I+CD8+ T cells after incubating T cells with vehicle- or VitE-treated BMDCs-derived EVs and immunofluorescent staining with CD8 and pMHC-I antibodies. EVs were collected from DiO-labelled and OVA-loaded BMDCs.
D, Flow cytometry analysis of the proliferation of OT-1 T cells cultured with the addition of EVs derived from OVA-loaded vehicle-DC-EVs or VitE-DC-EVs (left panel). IFN-γ production by OT-1 T cells with the indicated treatment was measured by ELISA (right panel, n=5).
E, Schema of adjacent T cell activation by cell-cell contact with VitE-treated DCs or sgPtpn6-transfected DCs and of distal T cell activation via pMHC-I expressing DC-EVs.
F, Growth of EO771-OVA mammary tumors and survival of mice treated with PBS (Vehicle), or DC-EVs (30 μg/mouse) isolated from DCs pretreated with vehicle (vehicle-DC-EVs), or with VitE (VitE-DC-EVs) (n=6/group).
G, Growth of EO771-OVA mammary tumors and survival of mice treated with ICT (anti-PD1, 200μg/mouse by i.p. at day 7, 10, 13) in combination with PBS, or DC-EVs (30 μg/mouse) isolated from DC2.4 cells transfected with sgCtrl (sgCtrl-DC2.4-EVs), or with sgPtpn6 (sgPtpn6-DC2.4-EVs) by i.v. at day 2, 9, and 16 (n=6/group).
H and I, Growth curves of subcutaneous B16-GMCSF-OVA tumors in mice treated with vehicle, sgCtrl-DC2.4-EVs or sgPtpn6-DC2.4-EVs (30μg/mouse) by i.v. at day 2, 9, and 16 (H, n = 6/group) and treated with ICT (anti-PD1) in combination with vehicle, sgCtrl-DC2.4-EVs or sgPtpn6-DC2.4-EVs (I, n=6/group).
J, Flow cytometry analysis and quantification of OVA-tetramer+CD8+ T cells in B16-GMCSF-OVA tumors from the 3 mouse groups described in I (n=6/group).
Error bars, s.e.m. Two-sided Student’s t-test (B, D). One-way ANOVA with Tukey’s multiple comparison post-test (F tumor volume, G tumor volume, H, I, and J). Two-sided log-rank test (F, G, survival curves). The statistical significance is defined by P-value: ns, no significant. *P < 0.05, **P < 0.01, ***P < 0.001.