Abstract
Background.
Persons with multimorbidity (≥2 chronic conditions) face increased risk of poor health outcomes, especially as they age. Psychosocial factors such as social isolation, chronic stress, housing insecurity, and financial insecurity have been shown to exacerbate these outcomes, but are not routinely assessed during the clinical encounter. Our objective was to extract these concepts from chart notes using natural language processing and predict their impact on healthcare utilization for patients with multimorbidity.
Methods.
A cohort study to predict the 1-year likelihood of hospitalizations and ED visits for patients 65+ with multimorbidity with and without psychosocial factors. Psychosocial factors were extracted from narrative notes; all other covariates were extracted from EHR data from a large academic medical center using validated algorithms and concept sets. Logistic regression was performed to predict likelihood of hospitalization and ED visit in the next year.
Results.
In all, 76,479 patients were eligible; the majority were white (89%), 54% were female, with mean age 73. Those with psychosocial factors were older, had higher baseline utilization, and more chronic illnesses. The four psychosocial factors all independently predicted future utilization (ORs=1.27 to 2.77, c-statistic=0.63). Accounting for demographics, specific conditions, and previous utilization, 3 of 4 of the extracted factors remained predictive (ORs= 1.13 to 1.86) for future utilization. Compared to models with no psychosocial factors, they had improved discrimination. Individual predictions were mixed, with social isolation predicting depression and morbidity; stress predicting atherosclerotic cardiovascular disease onset; and housing insecurity predicting substance use disorder morbidity.
Discussion.
Psychosocial factors are known to have adverse health impacts, but are rarely measured; using natural language processing, we extracted factors that identified a higher risk segment of older adults with multimorbidity. Combining these extraction techniques with other measures of social determinants may help catalyze population health efforts to address psychosocial factors to mitigate their health impacts.
Keywords: multimorbidity, psychosocial, biopsychosocial, natural language processing
Introduction
Over one-fourth of Americans, and three-fourths of those over 65, live with multimorbidity (≥2 chronic conditions).1, 2 Their care accounts for two-thirds of healthcare spending in the United States.3 However, chronic illnesses alone are often not the major contributor to poor health outcomes; rather, social and behavioral factors contribute significantly to worsened quality of life, increased disability, exacerbations of illness, and premature mortality.4 These psychosocial complexities (e.g., social isolation, chronic stress, financial insecurity) are also social determinants of health and contribute powerfully to racial/ethnic disparities in outcomes among older adults.5–8 There is growing awareness of the importance of understanding and managing the most impactful constructs – which we term psychosocial vital signs – in conjunction with biomedical problems that patients develop.9, 10
From the biopsychosocial perspective,11 uncovering the interconnections, overlap, and causal pathways between biomedical, psychological, and social processes remain important gaps. As of yet, these represent untapped opportunities to identify best practices that address the specific and unique barriers to effective chronic disease management for older adults with complex care needs; these include those with multiple chronic illnesses12 Key psychosocial vital signs for this cohort – those related to illness complications and increased disability – include social isolation, chronic stress, financial insecurity, and housing, among others. These factors are related to the onset of and impact from multimorbidity and affect health and well-being over the lifespan.5–8 For example, there is strong evidence regarding the importance of addressing financial and food insecurity in effective glycemic management13–17, and reducing rates of avoidable and intensive levels of health care utilization.14, 18 For chronic stress, the relationship may be the precipitating factor for increases to morbidity, especially in atherosclerotic disease.19–25
Despite their relationship to morbidity, structured assessment of these concepts is infrequent in clinical care. Individual social condition codes using ICD10 and validated questionnaires have shown significant promise in assessing social and psychological constructs, but their implementation outside of research settings is still suboptimal. The National Academy of Medicine assessed behavioral and social constructs that were feasible to collect, available, and scientifically acceptable,26 and several groups have tried to implement collection of these constructs in care settings.27 Initial results are encouraging but limited28–30: entering and reviewing structured data has a significant cost in the time of medical professionals. Without reliable data, overburdened clinical teams cannot know when it is crucial to collect or obtain this information and when to include it in care decisions. Areal or geographic measures of social determinants have value, but lack specificity in individual prediction.31 However, we and others find these vital signs embedded in narrative notes. Batra reviewed 82 studies using natural language processing to extract SDOH from text sources, demonstrating a burgeoning literature for extraction, but limited information about application.32 Greenwald et al. demonstrated that one could mine the narrative notes using simple keywords and consistently find a number of social and behavioral issues in inpatients;33 and Feller reports success in detecting these concepts in HIV patients.34 We previously found key psychosocial vital signs – housing insecurity, financial insecurity, social isolation, and chronic stress - could be extracted with high precision from clinical notes across older adults with multimorbidity.35 The relationship between these extracted variables and onset of and exacerbations of illness, however, is unclear compared to validated instruments.
The goal of this paper was to extend our previous work in extracting these psychosocial concepts to determine their utility in predicting exacerbations of illness for people who already were at risk from their comorbidities – as measured by unplanned hospitalizations and ED visits - with and without other standard predictors; and to test specific hypotheses for how these psychosocial factors may lead to increased exacerbations through pre-defined analyses.
Methods.
Study Design.
We completed a cohort study to understand the benefit of extracting 4 key psychosocial vital signs on prediction of future utilization within a health system. All data were extracted from the Electronic Health Record (EHR) as either structured (from discrete clinical fields) or unstructured (from narrative notes) data.
Population.
Figure 1 demonstrates the inclusion for the cohort; patients were part of the initial population if they were seen during 2016–2017 (N=665,818), were 65 and older (N=212,448), and had 2 or more chronic conditions recorded (N=76,479) at Oregon Health & Science University, a large academic health system.
Inclusion criteria and selection.
Besides age > 65 by 1/1/2017, patients had to be seen in the qualifying visit (baseline) year and the prediction year; the majority of these visits were ambulatory care visits. Patients also had to have 2 or more chronic diseases that are frequently included in operationalizing multimorbidity reflected on their problem list36, 37 for initial inclusion. Patients were excluded if they died before the end of their baseline year.
Feature extraction.
Pre-defined features were extracted from the EHR based their inclusion in previous models predicting outcomes. These included: 1) Demographics, including age, race, ethnicity, and biological sex; 2) individual Chronic Conditions, 3) Multimorbidity using Wei et al’s Multimorbidity-Weighted Index (MWI);38, 39 4) psychosocial vital signs, extracted both by ICD10 codes and through Natural Language Processing; and 5) Utilization (primary outcomes of hospitalizations and ED visits). Race and ethnicity were coded together; due to sample size, we first coded all persons selecting Black race as Black, then those selecting Hispanic ethnicity as Hispanic, then those selecting White race as White, then combining all others. Twelve of 76,000 patients selected Black and Hispanic and were coded as Black.
For Chronic Conditions, we selected 35 conditions previously known to be predictive of unplanned utilization and death in various models. We defined these conditions using standard value sets of ICD10 codes extracted from the Value Set Authority Center where possible. The list of value sets for each condition are provided in the Supplement Digital Content (1).
The four selected Psychosocial factors were extracted two ways: first, by using a set of ICD10 codes and structured data questionnaires; and then, using Natural Language Processing using lexical association expansion starting with regular expressions. The full detail is described elsewhere;35 in brief, the authors used an iterative process using ‘seeds’, or words and phrases likely to indicate the feature. For instance, housing insecurity used ‘housing insecurity’, ‘shelter’, ‘no housing’, ‘on the street’ for an initial run. An unsupervised learning approach based on Google’s word2vec (https://code.google.com/p/word2vec/) was used to learn word embeddings and identify other phrases more common in notes with the original phrases. Evaluation of the seeds is done using an information retrieval approach based on Term Frequency-Inverse Document Frequency (TF-IDF), Relevance, and Inverse Patient Frequency. Validation of the seeds was completed until Precision, or Positive Predictive Value (TruePositives / All Retrieved Documents) was > .90.
Multimorbidity measure.
To simplify the analyses and avoid overfitting, we implemented the Wei Multimorbidity Weighted Index measures. The MWI is a person-centered measure that weights chronic conditions based on their impact on the Short Form-36 physical functioning scale, generating negative coefficients. For ease of interpretation, we used the absolute value (positive coefficients) of the index in a set of predictive models which represents both the burden of chronic disease and physical functioning. In the MWI, chronic conditions (all except 4) are also carried forward to help decrease the underestimation of multimorbidity.
Analyses.
Prediction of subsequent (‘outcome’) year hospitalization and ED visits was the primary analytic goal. Descriptive analysis comparing those with and without the psychosocial factors was performed first. Then, planned logistical analyses were performed in the order listed in Table 1; we evaluated the models using likelihood ratios, c-statistics, and goodness of fit for discrimination and calibration.40–42 We limited features to avoid overfitting; in general, we had more than 100 observations per feature, a rough estimate for adequate sample size.43 The first analyses used no psychosocial vital signs, focusing on demographics, utilization, chronic conditions, and the weight comorbidity score. In the second stage, psychosocial factors were added to the model first, then each set of features (demographics, utilization, multimorbidity scores) were added consecutively. For conditions, a validated index from Wei were used in the final models in lieu of individual diagnoses. Finally, specific, predefined models were used to test individual hypotheses for the value of psychosocial measurements. These included the set in Table 1, focused on the known relationship between chronic stress and atherosclerotic cardiovascular disease (ASCVD) events; financial insecurity, housing insecurity, and onset of or worsening of diabetes; depression and social isolation together leading to worse outcomes; and others.
Table 1.
Pre-planned analyses
| Analysis | Rationale | Model |
|---|---|---|
| I.0 Prediction of utilization without psychosocial vital signs | Demographics, prior utilization, and multimorbidity predict future utilization. | ED / Hospitalizations predicted by all features except psychosocial vital signs. |
| I.1 All psychosocial vital signs to predict utilization | Psychosocial factors are known to exacerbate chronic illnesses. | ED / Hospitalizations predicted by all psychosocial vital signs and other features ; compare to I.0 |
| I.2 Diabetes morbidity and financial insecurity, housing insecurity | Patient costs for caring for diabetes have increased, and diabetes requires consistent places to store medication, leading to diabetes exacerbations | Predict ED / hospitalizations with housing insecurity and financial insecurity |
| I.3 ASCVD and Chronic stress | Chronic stress increases the risk of ASCVD outcomes | Predict ED/hospitalizations in patients with chronic stress and ASCVD |
| I.4 Depression and Social isolation | Social isolation and mood disorders generate more exacerbations together | Predict ED/hospitalizations in patients with social isolation and depression |
| I.5 Substance Use and housing insecurity | The combination of substance use and housing insecurity worsens outcomes | Preduct ED/hospitalizations in patietnts with substance use and housing insecurity |
ASCVD = Atherosclerotic Cardiovascular Disease; ED=Emergency Department
Sensitivity analyses.
We compared the logistic regression models with and without the psychosocial factors to see the relative model improvement (I.0 vs. I.1), and performed forwards and backwards stepwise inclusion to understand the impact of individual factors on the models. We also included patients with missing data in sensitivity models; these were coded as ‘missing’ for categorical variables or 0 for continuous variables.
Results.
In all, 76,479 patients met the initial criteria of 65 or older with 2 or more chronic conditions and one or more visits in 2016 or 2017. Excluding the 2,139 that died during their baseline year, 74,340 patients remained; 21,367 (27.9%) had one or more Psychosocial factors extracted from their chart. Validation of the extraction was presented previously; the average precision (or Positive Predictive Value) of the terms was .91.35 Table 2 presents the baseline characteristics of those with and without psychosocial factors. Patients with extracted psychosocial factors were much more likely to be hospitalized in prior years, more likely to have ED visits, but equally likely to have office visits. This indicates psychosocial vital signs are concurrent predictors of outcomes. They were similar by race (predominantly white) and sex (55% female) but those with psychosocial factors were slightly older; 56% of patients with no psychosocial factors were less than 76 versus 49% of those with psychosocial factors. Patients with psychosocial factors were also more likely to have hospitalizations, ED visits, or die in the outcome year.
Table 2.
Demographics of included patients by psychosocial factor presence
| No PS Factors N (%) |
1+ PS Factor N (%) |
|
|---|---|---|
| Total | 55,112 (100%) | 21,367 (100%) |
| Age | ||
| 66–70 | 17,435 (31.62%) | 3,824 (17.90%) |
| 71–75 | 13,498 (24.49%) | 6,775 (31.71%) |
| 76–80 | 10,041 (18.22%) | 4,761 (22.28%) |
| 81–85 | 6,570 (11.92%) | 2,889 (13.52%) |
| 86–90 | 4,084 (7.41%) | 1,698 (7.95%) |
| 91+ | 3,494 (6.34%) | 1,420 (6.65%) |
| Race/ethnicity | ||
| American Indian/Alaska Native | 297 (.54%) | 108 (.48%) |
| Asian | 1,488 (2.7%) | 599 (2.8%) |
| Black | 639 (1.16%) | 270 (1.26%) |
| Hispanic | 1,180 (2.14%) | 449 (2.1%) |
| Multiracial | 360 (.65%) | 151 (.71%) |
| Other | 1,604 (2.91%) | 420 (1.97%) |
| Pacific Islander | 106 (.19%) | 31 (.15%) |
| White | 49,438 (89.70%) | 19,344 (90.53%) |
| Female Sex | 29844 (54.15%) | 11,807 (55.26%) |
| Psychosocial factors | ||
| Chronic Stress | N/A 0 (0%) | 15,734 (73.64%) |
| Social isolation | 0 (0%) | 559 (2.62%) |
| Housing insecurity | 0 (0%) | 4,338 (20.30%) |
| Financial insecurity | 0 (0%) | 7,117 (33.31%) |
| Baseline utilization | ||
| 1+ Hospitalization | 4,987 (9.05%) | 7281 (34.08%) |
| 1+ ED visit | 2,294 (4.2%) | 2,909 (14.38%) |
| 1+ Office Visit | 51,607 (93.64%) | 20121 (94.17%) |
| Year before baseline utilization | ||
| 1+ Hospitalization | 2,356 (4.27%) | 1,616 (7.56%) |
| 1+ ED Visit | 1,083 (1.97%) | 1,310 (6.13%) |
| 1+ Office Visit | 26,617 (48.3%) | 12,303 (57.58%) |
| Most Frequent Diagnoses | ||
| Hypertension | 14772 (27.3%) | 8434 (41.69%) |
| Cancer | 14143 (26.14%) | 7021 (34.71%) |
| Hyperlipidemia | 8189 (15.13%) | 5078 (25.10%) |
| Arthritis | 6927 (12.80%) | 4419 (21.84%) |
| Dorsopathy | 5597 (10.34%) | 3880 (19.18%) |
| Depression | 4102 (7.58%) | 3268 (16.16%) |
| Diabetes | 6730 (12.44%) | 3135 (15.5%) |
| CAD | 4031 (7.45%) | 3035 (15%) |
| Osteoporosis | 3454 (6.38%) | 2723 (13.46%) |
| Anxiety | 2500 (4.62%) | 2413 (11.93%) |
| Severe Psychiatric illness | 1960 (3.62%) | 2196 (10.86%) |
| Multimorbidity-weighted index (Mean ± SD) | 7.7±6.8 | 12.1±9.5 |
| Outcomes in Prediction Year | ||
| 1+ Hospitalization | 2285 (4.15%) | 2480 (11.61%) |
| 1+ ED visit | 1687 (3.06%) | 2052 (9.6%) |
| Death* | 902 (1.6%) | 1028 (4.8%) |
| Hospitalization, ED visit or Death | 4320 (7.8%) | 4377 (20.48%) |
Deaths reported to health system only.
Analysis I.0 modeling results without psychosocial variables are demonstrated in the Supplemental Digital Content (2, Tables A.2 and A.3); models with demographics alone had poor performance (c-statistic=0.53) in predicting hospitalization. Adding individual chronic illnesses and previous utilization improved prediction substantially (c-statistic=0.70), butthe MWI score dramatically outperformed individual chronic illnesses (c-statistic=0.76). Similar results were seen for ED visits (demographics c=0.56; individual diagnoses c=0.76; MWI c=0.80).
Models with the psychosocial factors (I.1) results are shown in Table 3a (for hospitalizations) and 3b (for ED visits). The extracted psychosocial constructs alone were all significant predictors of future hospitalizations (PS-a, c-statistic 0.63), with Odds Ratios (ORs) that ranged from 1.38 (Social Isolation, 95% CI 1.07–1.79) to 2.26 (Chronic Stress, 95% CI 2.11–2.42). Adding race, age and the MWI increased performance to c-statistic 0.75. Individual conditions were modestly correlated with the psychosocial constructs (Table A.6) and a model with individual conditions instead of MWI and PS factors had worse performance (c-statistic 0.70). Adding previous utilization further increased the c-statistic (0.78) with previous hospitalizations and recent ED visits both significant. Thus, after accounting for previous hospitalizations and ED visits, knowing whether a patient was labeled as having chronic stress increased the odds of future hospitalization by 1.43. Goodness of fit was poor across all models (Hosmer-Lemeshow p < .01). Performance overall was only marginally improved from the models without psychosocial factors (Tables Appendix .2–3), with 1–2% of the cases more accurately classified.
Table 3.a.
Models to predict Hospitalizations including Psychosocial Factors
| Outcome | 1+ Hospitalization | ||
|---|---|---|---|
| Model | I.1.a Psychosocial factors alone | I.1.b PS, demographics, and MWI | I.1.c. PS, demographics, prior utilization, MWI |
| Likelihood Ratio (df) | 1183.4 (4) | 1827.7 (17) | 3649.9 (21) |
| C-stat | 0.63 | 0.75 | 0.77 |
| Hosmer-Lemeshow p | <.0001 | <.0001 | <.0001 |
| Variables | |||
| Psychosocial factors | |||
| Homelessness | 2.09 (1.89–2.32) * | 1.79 (1.61–1.99) * | 1.53 (1.37–1.71) * |
| Social isolation | 1.38 (1.07–1.79) * | 1.13 (0.86–1.48) | 1.02 (0.77–1.36) |
| Chronic Stress | 2.26 (2.11–2.42) * | 1.72 (1.6–1.85) * | 1.43 (1.33–1.55) * |
| Financial insecurity | 1.64 (1.49–1.79) * | 1.54 (1.4–1.69) * | 1.36 (1.24–1.5) * |
| Age (ref 66–70) | |||
| 71–75 | 0.82 (0.75–0.89) * | 0.84 (0.77–0.92) * | |
| 76–80 | 0.73 (0.66–0.81) * | 0.76 (0.69–0.84) * | |
| 81–85 | 0.77 (0.68–0.86) * | 0.8 (0.72–0.9) * | |
| 86–90 | 0.7 (0.61–0.8) * | 0.71 (0.62–0.82) * | |
| 91+ | 0.89 (0.77–1.03) | 0.88 (0.76–1.02) | |
| Race/ethnicity (ref White) | |||
| Native American | 0.94 (0.6–1.46) | 0.87 (0.56–1.36) | |
| Asian | 0.91 (0.74–1.11) | 0.92 (0.75–1.13) | |
| Black | 1.03 (0.78–1.34) | 1.01 (0.77–1.32) | |
| Hispanic | 1.05 (0.84–1.3) | 1.01 (0.81–1.26) | |
| Multiracial | 1.1 (0.77–1.58) | 1.08 (0.75–1.55) | |
| Pacific Islander | 1.13 (0.56–2.28) | 1.08 (0.53–2.22) | |
| Female | 0.85 (0.8–0.91) * | 0.85 (0.8–0.91) * | |
| Multimorbidity weighted index | 1.08 (1.07–1.08) * | 1.07 (1.07–1.07) * | |
| Hospitalization before baseline | 1.12 (1.05–1.19) * | ||
| … during baseline | 1.51 (1.45–1.57) * | ||
| Previous ED visit | 1.02 (0.95–1.1) | ||
| … during baseline | 1.15 (1.1–1.2) * |
For ED visit prediction, model performance was slightly improved over hospitalizations (c-statistics=.65-.81). Psychosocial factors alone remained highly predictive (ORs 1.27–2.77); adding race, age, sex and comorbidity scores increased the model performance with moderate impact on PS odds ratios. The exception was social isolation, which no longer significantly predicted outcomes. Older age (86+) and Black and Hispanic race/ethnicity increased odds of ED visits. The MWI remained highly predictive, with each 1 point increase associated with an increased odds ratio between 1.05–1.07. The final models with utilization showed a further increase in prediction, with a 2 year history of ED visits useful in prediction (OR 1.4 for year prior to baseline; OR 2.2 for baseline) but only the baseline years’ hospitalizations (OR 1.06) were predictive.
Individual predictions.
Impact for specific combinations of conditions and psychosocial factors The pre-planned individual prediction models are shown in Table 4 and in the Supplemental Digital Content (2, Tables A.4–A.5). For patients with diabetes at baseline, hospitalizations were slightly better predicted than the overall model with similar odds ratios. ED visits were not better predicted. For patients with ASCVD, chronic stress was a strong predictor of hospitalization and ED visits alone but moderate in adjusted models. Social isolation was a predictor with depression for ED and hospitalization unadjusted models, but it did not reach significance in the adjusted models. Housing insecurity and substance use disorder were strong predictors of ED and hospitalization in unadjusted but not adjusted models; and mental health scores predicted increased, not decreased, office visits.
Table 4.
Pre-specified models with conditions and psychosocial vital signs
| Individual | I.2 Diabetes | I.3 ASCVD | I.4 Depression | I.5 Substance Use | ||||
|---|---|---|---|---|---|---|---|---|
| Hospitalization | ED | Hospitalization | ED | Hospitalization | ED | Hospitalization | ED | |
| C-statistic | 0.6 | 0.6 | 0.63 | 0.66 | 0.53 | 0.56 | 0.55 | 0.56 |
| Condition | Diabetes | ASCVD | Depression | Substance Use | ||||
| Condition result | 2.54 (2.31–2.79) * | 2.80 (2.53–3.1) * | 2.45 (2.29–2.62) | 2.85 (2.66–3.06) * | 2.59 (2.02–3.33) * | 2.49 (1.93–3.22) * | 3.1 (2.82–3.41) * | 3.18 (2.88–3.52) * |
| PS Factors | 1. Housing, 2. Financial | Chronic Stress | Social isolation | Housing | ||||
| PS Result 1 | 2.18 (2.01–2.36) * | 1.67 (1.52–1.84) * | 1.96 (1.82–2.1) * | 2.24 (2.08–2.42) * | 1.52 (1.39–1.67) * | 2.4 (2.2–2.62) * | 1.76 (1.5–2.07) * | 2.65 (2.28–3.08) * |
| PS Result 2 | 1.52 (1.41–1.64) * | 1.73 (1.60–1.99) * | ||||||
| Full: Likelihood Ratio | 720.6 (19) | 730.3 (19) | 674.7 (21) | 782.0 (21) | 359.2 (21) | 542.2 (21) | 105.2 (21) | 213.6 (21) |
| C-stat | 0.76 | 0.77 | 0.71 | 0.76 | 0.73 | 0.74 | 0.73 | 0.79 |
| Hosmer-Lemeshow p | <.0001 | <.0001 | 0.0683 | <.0001 | 0.003 | 0.067 | 0.09 | 0.06 |
| Homelessness | 1.63 (1.3–2.03) * | 1.48 (1.16–1.89) * | 1.39 (1.16–1.66) * | 1.59 (1.41–1.79) * | 1.43 (1.11–1.84) * | 1.17 (0.91–1.5) | 1.41 (0.92–2.16) | 1.2 (0.78–1.85) |
| Social isolation | 1.01 (0.65–1.57) | 1.25 (0.93–1.67) | 1.27 (0.85–1.9) | 1.02 (0.68–1.53) | 1.22 (0.5–2.99) | 1.12 (0.45–2.79) | ||
| Chronic Stress | 1.68 (1.48–1.92) * | 2.08 (1.92–2.26) * | 1.5 (1.24–1.81) * | 1.86 (1.56–2.22) * | 1.68 (1.18–2.4) * | 1.27 (0.89–1.81) | ||
| Financial insecurity | 1.74 (1.43–2.11) * | 1.49 (1.19–1.85) * | 1.24 (1.05–1.46) * | 1.18 (1.06–1.32) * | 1.48 (1.18–1.85) * | 1.2 (0.96–1.5) | 0.66 (0.42–1.04) | 1.05 (0.68–1.62) |
| Age (ref 66–70) | ||||||||
| 71–75 | 0.83 (0.68–1.02) | 0.82 (0.66–1.02) | 0.96 (0.8–1.15) * | 0.76 (0.69–0.84) * | 0.88 (0.7–1.11) | 0.75 (0.6–0.94) * | 1.1 (0.74–1.63) | 0.57 (0.37–0.88) * |
| 76–80 | 0.99 (0.8–1.23) | 0.85 (0.67–1.08) | 0.9 (0.74–1.09) * | 0.78 (0.7–0.87) * | 0.74 (0.56–0.98) * | 0.9 (0.7–1.15) | 0.75 (0.43–1.3) | 0.6 (0.35–1.03) |
| 81–85 | 1.1 (0.86–1.4) | 0.93 (0.72–1.22) | 0.91 (0.74–1.12) | 0.88 (0.78–0.99) * | 1.07 (0.79–1.45) | 0.96 (0.72–1.29) | 0.52 (0.25–1.08) | 0.96 (0.54–1.7) |
| 86–90 | 0.88 (0.63–1.21) | 1.13 (0.82–1.56) | 0.75 (0.59–0.96) * | 1.21 (1.06–1.38) * | 1.13 (0.78–1.64) | 1.43 (1.03–1.99) * | 0.99 (0.43–2.29) | 1.61 (0.8–3.24) |
| 91+ | 1.29 (0.93–1.79) | 1.32 (0.94–1.85) | 0.88 (0.69–1.13) | 1.43 (1.24–1.64) * | 1.03 (0.68–1.56) | 1.1 (0.75–1.61) | 0.81 (0.27–2.4) | 1.64 (0.72–3.74) |
| Race/ethnicity (ref White) | ||||||||
| Native American | 0.81 (0.36–1.83) | 0.37 (0.11–1.23) | 0.92 (0.43–1.98) | 0.84 (0.51–1.4) | 0.77 (0.23–2.59) | 0.16 (0.02–1.25) | 0.54 (0.07–4.44) | n/a |
| Asian | 0.71 (0.46–1.08) | 0.9 (0.59–1.36) | 1.27 (0.89–1.81) | 1.05 (0.84–1.3) | 0.71 (0.37–1.37) | 1.07 (0.63–1.82) | n/a | 2.56 (0.53–12.32) |
| Black | 0.91 (0.55–1.51) | 1.49 (0.94–2.37) | 1.25 (0.81–1.92) | 1.76 (1.36–2.27) * | 1.29 (0.67–2.48) | 1.35 (0.72–2.53) | 1 (0.4–2.47) | 3.08 (1.51–6.31) * |
| Hispanic | 0.98 (0.68–1.4) | 1.34 (0.94–1.9) | 1.15 (0.77–1.7) | 1.33 (1.07–1.65) * | 1.05 (0.58–1.89) | 1.18 (0.69–2) | 0.25 (0.03–1.95) | 0.55 (0.12–2.42) |
| Multiracial | 0.82 (0.38–1.77) | 1.42 (0.71–2.88) | 1.01 (0.51–1.99) | 1.08 (0.71–1.62) | 0.6 (0.21–1.76) | 1.04 (0.43–2.49) | 0.83 (0.18–3.74) | 0.76 (0.18–3.35) |
| Pacific Islander | 1.22 (0.46–3.26) | 1.13 (0.38–3.33) | 1.57 (0.58–4.26) | 1.26 (0.59–2.69) | 2.45 (0.29–20.47) | 2.24 (0.27–18.8) | 15.38 (1.08–219.8) | 0.68 (0.01–95.58) |
| Female | 0.87 (0.75–1.01) | 1.01 (0.86–1.18) | 0.9 (0.79–1.02) | 1.02 (0.95–1.1) | 0.86 (0.72–1.04) | 0.99 (0.83–1.18) | 0.9 (0.64–1.26) | 0.93 (0.67–1.29) |
| hospitalization before baselin | 1.23 (1.1–1.36) * | 0.88 (0.77–1.01) | 1.16 (1.07–1.26) * | 1.04 (0.96–1.12) | 1.28 (1.13–1.46) * | 0.9 (0.77–1.06) | 1.16 (0.94–1.43) | 1.05 (0.84–1.31) |
| … during baseline | 1.65 (1.53–1.78) * | 1.19 (1.1–1.3) * | 1.34 (1.26–1.42) * | 1.07 (1.02–1.12) * | 1.48 (1.33–1.64) * | 1.18 (1.06–1.31) | 1.55 (1.32–1.82) * | 1.15 (0.97–1.37) |
| Previous ED visit | 0.9 (0.77–1.06) | 1.46 (1.24–1.72) * | 1.08 (0.96–1.21) * | 1.57 (1.44–1.71) * | 1.01 (0.89–1.14) | 1.08 (0.94–1.24) | 0.97 (0.83–1.12) | 0.98 (0.86–1.11) |
| … during baseline | 1.21 (1.11–1.31) * | 2.24 (2.01–2.49) * | 1.24 (1.16–1.33) * | 2.37 (2.25–2.5) * | 1.08 (0.98–1.2) | 1.97 (1.76–2.19) | 1.11 (1.02–1.21) * | 2.08 (1.76–2.46) * |
| Wei score | 1.07 (1.06–1.08) * | 1.07 (1.05–1.08) * | 1.05 (1.04–1.06) * | 1.03 (1.01–1.04) * | 1.05 (1.04–1.07) * | 1.05 (1.04–1.06) * | 1.03 (1.01–1.06) * | 1.03 (1.01–1.06) * |
Discussion
Psychosocial factors extracted from natural language processing were predictive of unplanned hospitalizations and ED visits, even when accounting for multimorbidity with a validated score, age, sex, race, and previous utilization. This confirms the value of psychosocial factors in older adults with multimorbidity as well as the value of concepts extracted from narrative notes. The net benefit to the models, however, was small compared to those without psychosocial factors, with only 1–2 / 100 additional patients accurately predicted. Social isolation was infrequently recorded in narrative notes and its effect was limited. Individual condition models with predefined populations showed strong prediction for unadjusted models predicting onset of conditions associated with the psychosocial factors (stress – ASCVD; social isolation – depression), but mixed results in focused models adjusted for other factors. For instance, patients with diabetes had better prediction models for hospitalizations from housing insecurity and financial insecurity, but not for depression and social isolation. Thus, adding psychosocial variables for patients with higher multimorbidity appeared to be a more consistently powerful predictor of utilization than attempting to use individual conditions; this lends itself to use in more comprehensive models of care management and coordination.44, 45
Previous work has looked at the benefits of collecting social determinants of health as psychosocial factors. A review showed increasing rates of structured collection of social determinants in EHRs showed promise in use in prediction and quality improvement, but many barriers exist, including lack of agreement on how, when, and why to collect the data.46–48 When collected, structured factors do predict important outcomes; recent work shows structured documentation of social determinants through ICD-10 codes was predictive of ED visits.
Others have explored the use of automated extraction of terms related to psychosocial factors. Bejan et al. demonstrated an accurate method of housing insecurity and adverse childhood experience identification over time;49 Feller et al. successfully extracted psychosocial terms in patients with HIV;47 and Newman-Griffis and Fosler-Lussier had high accuracy scores for extracted psychosocial and disability scores.50 A recent review identified 82 studies focused on EHR-based extraction of social determinants of health using NLP methodologies, demonstrating that most SDOH constructs can be extracted using similar rule-based approaches.32 Another recent review analyzed the literature related to the integration of social determinants into electronic health records and their impact on risk prediction applications, showing individual level SDOH outperformed areal measures like the Social Vulnerability Index.31 This article adds to this literature by providing evidence that psychosocial factors are useful in prediction even in the relatively short term; and providing comparative benchmarks for the relative gain in prediction overall and for specific use-cases. Given the cost of either individual data collection or extraction, focused efforts to collect/extract these factors and use them in models may make more sense than broad screening.
There are several limitations to this study. First, the data is limited to a single health system. Although the data are enhanced in key ways – deaths, ED visits, and hospitalization information is shared and stored from across the local area – the data are likely fragmented and missing key elements for certain patients. This means that the predictions may be partially attributed to volume of data available rather than the unique meaning of each concept. This is particularly important for the psychosocial factors which are not collected in a standard fashion but were extracted from notes; the times and reasons health professionals mention these factors are likely not at random, but tied to the frequency and interactions with the health care system. The diagnosis codes themselves, however, are also used in relation to health system visits, and are overrepresented in frequent visits. The modeling also showed poor goodness of fit; in part, this was due to the fact the two populations – with and without psychosocial factors – are quite different, yielding uneven probability distributions. Focusing on a subset of the population – older adults with multimorbidity – also likely skews the models. This segment, however, is both the fastest growing and most likely to show near term outcomes; the variability in these outcomes make additional factors, like psychosocial variables, particularly valuable to plan potential interventions. Others have used additional socio-economic and geocoded social determinants such as the Social Vulnerability Index51 and Area Deprivation Index;52 however, they may have limited effect when individual variables are available.53
Future work is needed to better integrate these approaches. Determining when and how to screen for /collect structured data for psychosocial factors is still unclear; some reports note that people may be reluctant to ask for help and care teams may see these factors as outside care. Automated extraction could further help decide when individual level collection would be helpful; understanding the sociotechnical aspects of these solutions would be crucial, however, to develop thoughtful interventions. Recent inclusion of these psychosocial vital signs in the health data standards known as the United States Core Data Interoperability definitions answers more of the how, but the burden of data collection remains. More careful work to segment the population most at-risk into potential actionable strategies and interventions is necessary as well, using both areal and individual factors; collecting data that does not yield action could worsen inequity and cause further frustration. Given the low additional benefit of psychosocial variables when a robust dataset is available, adding psychosocial variables should be tailored to the problem of interest. That is, if the psychosocial variables are hypothesized to affect outcomes, the models should be created to address the hypotheses directly. For instance, food insecurity and diabetes may be most impactful for patients on insulin or with severe diabetes. To move to action, better understanding of the causal frameworks for psychosocial factors in the context of multimorbidity is needed. Advancements in modeling are present to infer causality, but have only been applied to these factors in small studies. More rigorous and thorough work is needed.
In all, psychosocial concepts extracted from natural language processing of clinical notes were highly predictive of future utilization and death after rigorous adjustment for patient factors such as multimorbidity, physical functioning, and prior utilization. These approaches may help us understand the vast disparities in health care and enable us to move toward innovations to better address psychosocial needs and improve our systems of health and health care.
Supplementary Material
Figure 1.
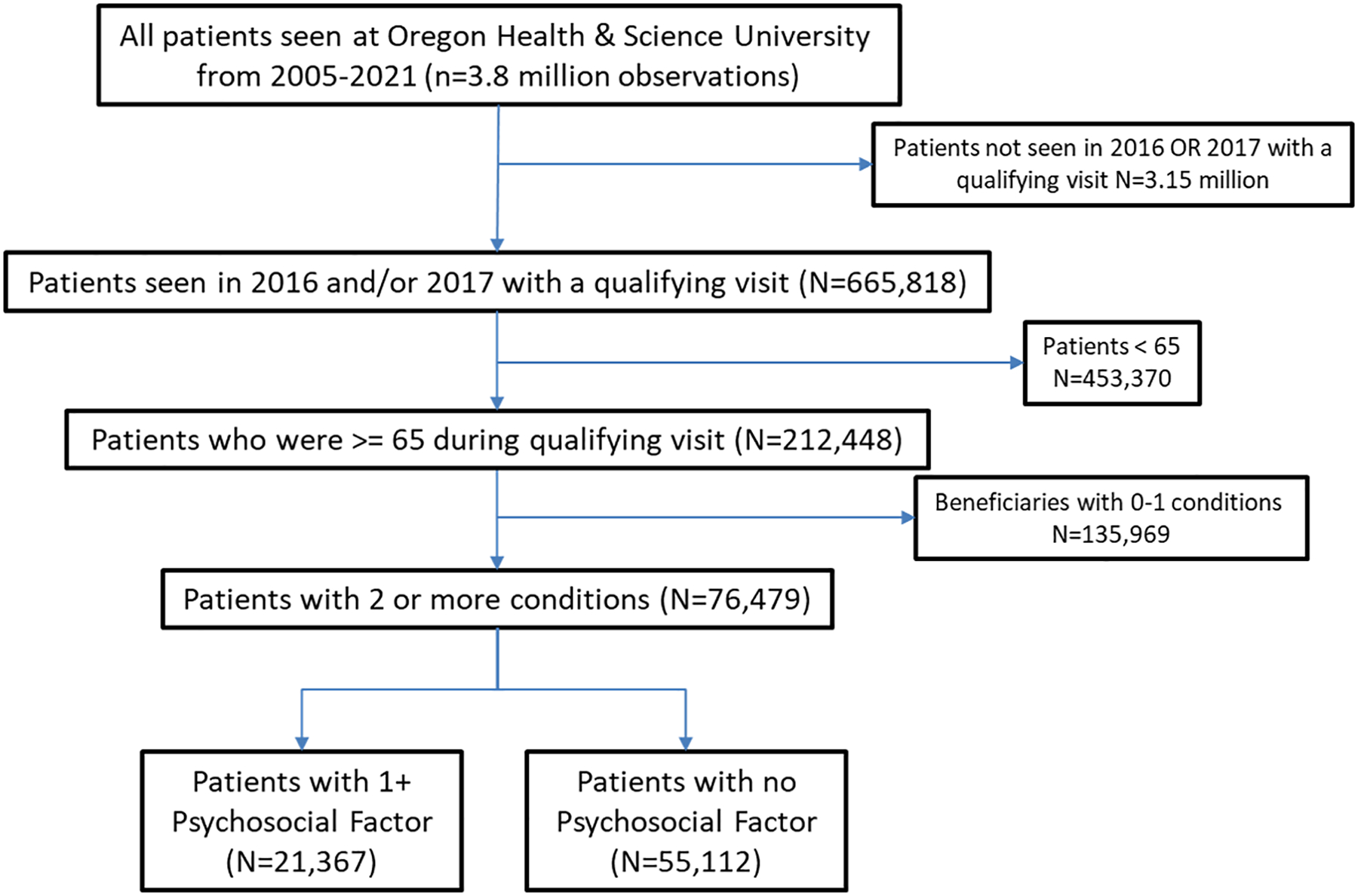
Table 3.b.
Prediction of Emergency Department visits at 1 year post-baseline.
| Outcome | 1+ ED visits | ||
|---|---|---|---|
| Model | I.1.a Psychosocial factors alone | I.1.b PS, demographics, and MWI | I.1.c. PS, demographics, prior utilization, MWI |
| Likelihood Ratio (df) | 1252.5 (4) | 2437.9 (17) | 3817.9 (21) |
| C-stat | 0.65 | 0.77 | 0.81 |
| Hosmer-Lemeshow p | 0.0036 | <.0001 | |
| Variables | |||
| Psychosocial factors | |||
| Homelessness | 2.24 (2.01–2.5) * | 1.92 (1.72–2.15) * | 1.48 (1.31–1.67) * |
| Social isolation | 1.53 (1.18–1.99) * | 1.2 (0.91–1.59) | 1.06 (0.78–1.44) |
| Chronic Stress | 2.77 (2.57–2.98) * | 2.09 (1.93–2.26) * | 1.73 (1.59–1.88) * |
| Financial insecurity | 1.27 (1.14–1.4) * | 1.19 (1.07–1.32) * | 1.12 (1–1.26) * |
| Age (ref 66–70) | |||
| 71–75 | 0.72 (0.65–0.79) * | 0.75 (0.68–0.83) * | |
| 76–80 | 0.68 (0.61–0.76) * | 0.73 (0.65–0.81) * | |
| 81–85 | 0.75 (0.66–0.85) * | 0.78 (0.69–0.89) * | |
| 86–90 | 1.04 (0.91–1.19) | 1.07 (0.93–1.23) | |
| 91+ | 1.43 (1.25–1.64) * | 1.36 (1.18–1.56) * | |
| Race/ethnicity (ref White) | |||
| Native American | 0.93 (0.57–1.53) | 0.84 (0.5–1.4) | |
| Asian | 1.01 (0.81–1.25) | 1.04 (0.84–1.29) | |
| Black | 1.83 (1.43–2.33) * | 1.65 (1.27–2.14) * | |
| Hispanic | 1.48 (1.21–1.83) * | 1.34 (1.08–1.67) * | |
| Multiracial | 1.19 (0.81–1.75) | 1.08 (0.72–1.63) | |
| Pacific Islander | 1.48 (0.72–3) | 1.18 (0.55–2.55) | |
| Female | 0.96 (0.89–1.03) | 0.98 (0.91–1.05) | |
| Multimorbidity weighted index | 1.08 (1.08–1.08) * | 1.07 (1.07–1.08) * | |
| Hospitalization before baseline | 0.91 (0.84–0.99) | ||
| … during baseline | 1.06 (1.01–1.11) * | ||
| Previous ED visit | 1.38 (1.27–1.51) * | ||
| … during baseline | 2.22 (2.11–2.34) * |
Footnotes
The authors do not report any conflicts of interest with this work.
References
- 1.Tinetti ME, Fried TR and Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA 2012; 307: 2493–2494. DOI: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salive ME. Multimorbidity in older adults. Epidemiologic Reviews 2013; 35: 75–83. 2013/02/02. DOI: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 3.Lochner KA and Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Preventing Chronic Disease 2013; 10: E61. 2013/04/27. DOI: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shippee ND, Shah ND, May CR, et al. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. Journal of Clinical Epidemiology 2012; 65: 1041–1051. 2012/08/23. DOI: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Despard M, Grinstein-Weiss M, Guo S, et al. Financial Shocks, Liquid Assets, and Material Hardship in Low- and Moderate-Income Households: Differences by Race. Journal of Economics, Race, and Policy 2018; 1: 205–216. DOI: 10.1007/s41996-018-0011-y. [DOI] [Google Scholar]
- 6.Marshall GL and Tucker-Seeley R. The association between hardship and self-rated health: does the choice of indicator matter? Annals of Epidemiology 2018; 28: 462–467. DOI: 10.1016/j.annepidem.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall GL, Kahana E, Gallo WT, et al. The price of mental well-being in later life: the role of financial hardship and debt. Aging & Mental Health 2020: 1–7. DOI: 10.1080/13607863.2020.1758902. [DOI] [PubMed] [Google Scholar]
- 8.Tucker-Seeley RD, Li Y, Subramanian SV, et al. Financial Hardship and Mortality among Older Adults Using the 1996–2004 Health and Retirement Study. Annals of Epidemiology 2009; 19: 850–857. DOI: 10.1016/j.annepidem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suls J and Rothman A. Evolution of the biopsychosocial model: prospects and challenges for health psychology. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 2004; 23: 119–125. 2004/03/11. DOI: 10.1037/0278-6133.23.2.119. [DOI] [PubMed] [Google Scholar]
- 10.Borrell-Carrio F, Suchman AL and Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Annals of family medicine 2004; 2: 576–582. 2004/12/04. DOI: 10.1370/afm.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel GL. The clinical application of the biopsychosocial model. The American journal of psychiatry 1980; 137: 535–544. 1980/05/01. DOI: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- 12.Fava GA and Sonino N. The biopsychosocial model thirty years later. Psychotherapy and psychosomatics 2008; 77: 1–2. 2007/12/19. DOI: 10.1159/000110052. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald N, Hromi-Fiedler A, Segura-Pérez S, et al. Food insecurity is related to increased risk of type 2 diabetes among Latinas. Ethnicity & disease 2011; 21: 328. [PMC free article] [PubMed] [Google Scholar]
- 14.Gucciardi E, Vahabi M, Norris N, et al. The intersection between food insecurity and diabetes: a review. Current nutrition reports 2014; 3: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gucciardi E, Vogt JA, DeMelo M, et al. Exploration of the relationship between household food insecurity and diabetes in Canada. Diabetes care 2009; 32: 2218–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holben DH and Pheley AM. Diabetes Risk and Obesity in Food-Insecure Households in Rural Appalachian Ohio. Preventing chronic disease 2006; 3. [PMC free article] [PubMed] [Google Scholar]
- 17.Seligman HK, Jacobs EA, Lopez A, et al. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes care 2012; 35: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seligman HK, Davis TC, Schillinger D, et al. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. Journal of health care for the poor and underserved 2010; 21: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nousen EK, Franco JG and Sullivan EL. Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology 2013; 98: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pervanidou P and Chrousos GP. Stress and obesity/metabolic syndrome in childhood and adolescence. International Journal of Pediatric Obesity 2011; 6: 21–28. [DOI] [PubMed] [Google Scholar]
- 21.VanItallie TB. Stress: a risk factor for serious illness. Metabolism-Clinical and Experimental 2002; 51: 40–45. [DOI] [PubMed] [Google Scholar]
- 22.Vitaliano PP, Scanlan JM, Zhang J, et al. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic medicine 2002; 64: 418–435. [DOI] [PubMed] [Google Scholar]
- 23.Cramm JM and Nieboer AP. Relationships between frailty, neighborhood security, social cohesion and sense of belonging among community‐dwelling older people. Geriatrics & gerontology international 2013; 13: 759–763. [DOI] [PubMed] [Google Scholar]
- 24.Gale CR, Westbury L and Cooper C. Social isolation and loneliness as risk factors for the progression of frailty: the English Longitudinal Study of Ageing. Age ageing 2017; 47: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strawbridge WJ, Shema SJ, Balfour JL, et al. Antecedents of frailty over three decades in an older cohort. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 1998; 53: S9–S16. [DOI] [PubMed] [Google Scholar]
- 26.Hripcsak G, Forrest CB, Brennan PF, et al. Informatics to support the IOM social and behavioral domains and measures. J Am Med Inform Assoc 2015; 22: 921–924. 2015/04/29. DOI: 10.1093/jamia/ocv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IOM. Capturing social and behavioral domains and measures in electronic health records: Phase 2. Washington, DC: Washington, DC: The National Academies Press, 2014. [PubMed] [Google Scholar]
- 28.Gold R, Cottrell E, Bunce A, et al. Developing Electronic Health Record (EHR) Strategies Related to Health Center Patients’ Social Determinants of Health. The Journal of the American Board of Family Medicine 2017; 30: 428–447. DOI: 10.3122/jabfm.2017.04.170046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazemore AW, Cottrell EK, Gold R, et al. “Community vital signs” : incorporating geocoded social determinants into electronic records to promote patient and population health. Journal of the American Medical Informatics Association : JAMIA 2015; 23: 407–412. DOI: 10.1093/jamia/ocv088. [DOI] [PubMed] [Google Scholar]
- 30.DeVoe JE, Bazemore AW, Cottrell EK, et al. Perspectives in Primary Care: A Conceptual Framework and Path for Integrating Social Determinants of Health Into Primary Care Practice. The Annals of Family Medicine 2016; 14: 104–108. DOI: 10.1370/afm.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Tan X and Padman R. Social determinants of health in electronic health records and their impact on analysis and risk prediction: A systematic review. J Am Med Inform Assoc 2020; 27: 1764–1773. DOI: 10.1093/jamia/ocaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patra BG, Sharma MM, Vekaria V, et al. Extracting social determinants of health from electronic health records using natural language processing: a systematic review. J Am Med Inform Assoc 2021; 28: 2716–2727. DOI: 10.1093/jamia/ocab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwald JL, Cronin PR, Carballo V, et al. A Novel Model for Predicting Rehospitalization Risk Incorporating Physical Function, Cognitive Status, and Psychosocial Support Using Natural Language Processing. Medical care 2016. 2016/09/16. DOI: 10.1097/mlr.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 34.Feller DJ, Bear Don’t Walk Iv OJ, Zucker J, et al. Detecting Social and Behavioral Determinants of Health with Structured and Free-Text Clinical Data. Appl Clin Inform 2020; 11: 172–181. 2020/03/05. DOI: 10.1055/s-0040-1702214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorr D, Bejan CA, Pizzimenti C, et al. Identifying Patients with Significant Problems Related to Social Determinants of Health with Natural Language Processing. Stud Health Technol Inform 2019; 264: 1456–1457. DOI: 10.3233/shti190482. [DOI] [PubMed] [Google Scholar]
- 36.Goodman RA, Posner SF, Huang ES, et al. Defining and Measuring Chronic Conditions: Imperatives for Research, Policy, Program, and Practice. Preventing Chronic Disease 2013; 10: E66. DOI: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei MY, Kabeto MU, Langa KM, et al. Multimorbidity and Physical and Cognitive Function: Performance of a New Multimorbidity-Weighted Index. The Journals of Gerontology: Series A 2018; 73: 225–232. DOI: 10.1093/gerona/glx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei MY, Luster JE, Ratz D, et al. Development, Validation, and Performance of a New Physical Functioning-Weighted Multimorbidity Index for Use in Administrative Data. J Gen Intern Med 2021. 2021/01/21. DOI: 10.1007/s11606-020-06486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei MY, Ratz D and Mukamal KJ. Multimorbidity in Medicare Beneficiaries: Performance of an ICD-Coded Multimorbidity-Weighted Index. J Am Geriatr Soc 2020; 68: 999–1006. 2020/01/10. DOI: 10.1111/jgs.16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010; 21: 128–138. DOI: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steyerberg E Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer; New York, 2010. [Google Scholar]
- 42.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York, 2013. [Google Scholar]
- 43.Riley RD, van der Windt D, Croft P, et al. Prognosis Research in Healthcare: Concepts, Methods, and Impact. OUP Oxford, 2019. [Google Scholar]
- 44.Dorr DA, Wilcox AB, Brunker CP, et al. The effect of technology-supported, multidisease care management on the mortality and hospitalization of seniors. J Am Geriatr Soc 2008; 56: 2195–2202. 2008/12/20. DOI: 10.1111/j.1532-5415.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 45.Samal L, Fu HN, Camara DS, et al. Health information technology to improve care for people with multiple chronic conditions. Health Serv Res 2021; 56 Suppl 1: 1006–1036. 20211005. DOI: 10.1111/1475-6773.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lasser EC, Kim JM, Hatef E, et al. Social and Behavioral Variables in the Electronic Health Record: A Path Forward to Increase Data Quality and Utility. Acad Med 2021; 96: 1050–1056. 2021/03/19. DOI: 10.1097/acm.0000000000004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feller DJ, Zucker J, Walk OBD, et al. Longitudinal analysis of social and behavioral determinants of health in the EHR: exploring the impact of patient trajectories and documentation practices. AMIA Annu Symp Proc 2019; 2019: 399–407. 2020/04/21. [PMC free article] [PubMed] [Google Scholar]
- 48.Hatef E, Rouhizadeh M, Tia I, et al. Assessing the Availability of Data on Social and Behavioral Determinants in Structured and Unstructured Electronic Health Records: A Retrospective Analysis of a Multilevel Health Care System. JMIR Med Inform 2019; 7: e13802. 2019/08/04. DOI: 10.2196/13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bejan CA, Angiolillo J, Conway D, et al. Mining 100 million notes to find homelessness and adverse childhood experiences: 2 case studies of rare and severe social determinants of health in electronic health records. J Am Med Inform Assoc 2018; 25: 61–71. DOI: 10.1093/jamia/ocx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman-Griffis D and Fosler-Lussier E. Automated Coding of Under-Studied Medical Concept Domains: Linking Physical Activity Reports to the International Classification of Functioning, Disability, and Health. Front Digit Health 2021; 3 2021/04/02. DOI: 10.3389/fdgth.2021.620828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention/ Agency for Toxic Substances and Disease Registry/ Geospatial Research A, and Services Program. CDC/ATSDR Social Vulnerability Index 2018 Database US. . https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html.2018. [Google Scholar]
- 52.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health 2003; 93: 1137–1143. DOI: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kostelanetz S, Di Gravio C, Schildcrout JS, et al. Should We Implement Geographic or Patient-Reported Social Determinants of Health Measures In Cardiovascular Patients. Ethn Dis 2021; 31: 9–22. 20210121. DOI: 10.18865/ed.31.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


