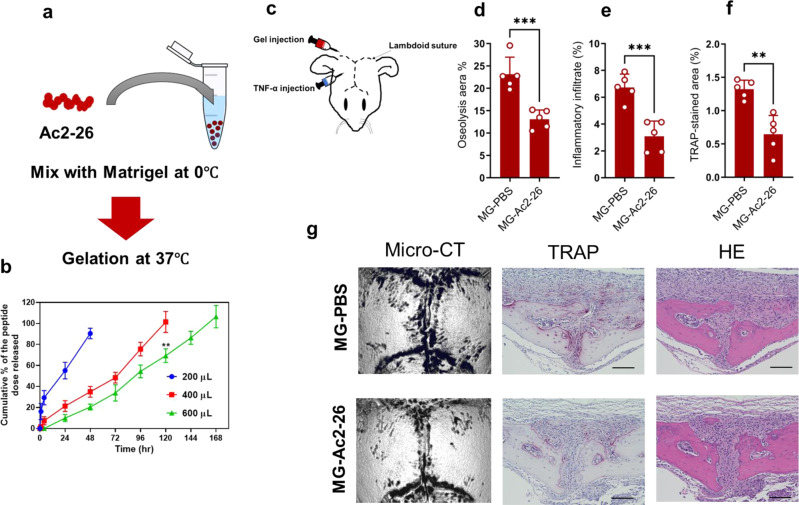
Fig. 8. Matrigel encapsulating Ac2–26 and its pharmacological potential for the treatment of pathological bone resorption induced by TNF-α.
a Schematic diagram showing the procedure for preparing the Matrigel encapsulating the Ac2–26 peptide. b In vitro release of the peptide from hydrogels containing different amounts of Matrigel at 37 °C and pH 7.4. The results represent the mean ± SEM of independent experiments. **p < 0.001 Significance was determined by the two-tailed Student’s t test. c Schematic diagram for the injection of gel and cytokines. d–g Therapeutic effect of Matrigel encapsulating Ac2–26 in a bone loss model induced by TNF-α administration. The MG-PBS was the control gel, and the MG-Ac2–26 is test gel. d Quantification of lytic area in calvarial bone tissues analyzed by micro-CT. e, f Quantification of TRAP-stained areas and inflammatory infiltrate in calvarial bone tissues. A significant difference between groups was determined by the two-tailed Student’s t test. *p < 0.05, **p < 0.001, ***p < 0.0001. Results represent the mean ± SEM of 5 mice. g Representative images for micro-CT and histological observations of bone sections stained by TRAP and H&E. Scale bar 100 µm. Source data are provided as a Source Data file.