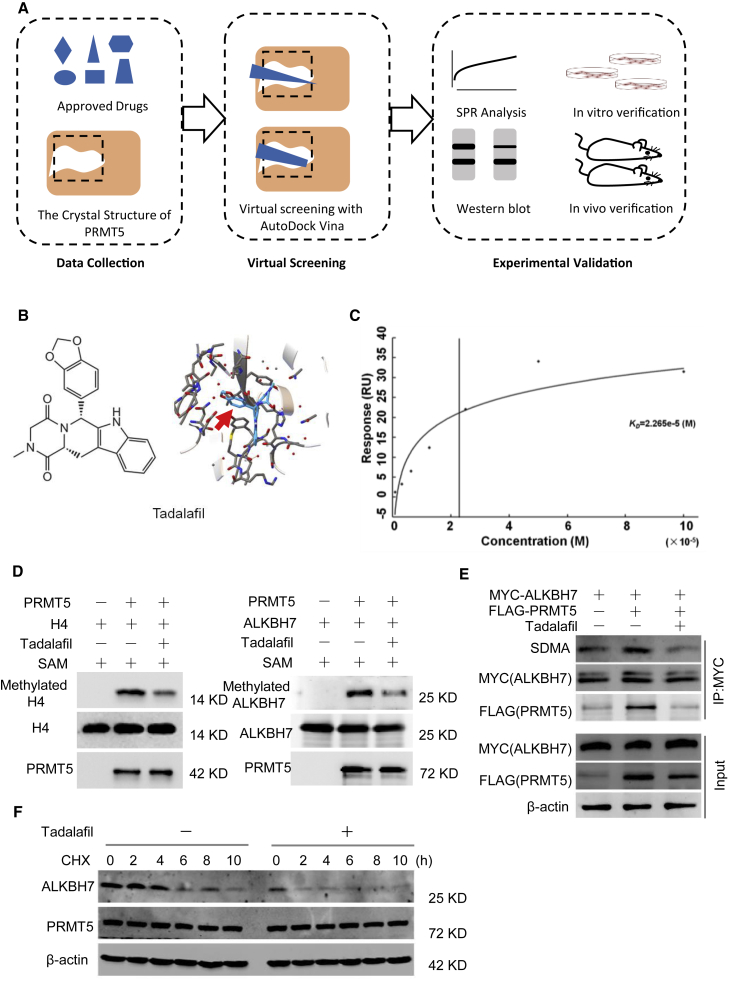
Figure 6.
Approved drug tadalafil is identified as a novel PRMT5-targeting inhibitor
(A) Structure-based virtual screening of PRMT5 inhibitors among 1,813 approved drugs. (B) Molecular structures and binding modules of the re-positioned approved drug tadalafil docked in the 3UA4 crystal structure of the PRMT5 protein. (C) SPR analysis to show the binding affinity of tadalafil with the purified PRMT5/MEP50 protein complex. Kd = 2.265 × 10−5 M. (D) In vitro methylation assay with recombinant FLAG-tagged PRMT5 and unlabeled SAM. The substrate was wild-type His-tagged ALKBH7, and the conditions were treatment with or without 100 μM tadalafil. The same blot was stripped and re-probed with an anti-ALKBH7 antibody, showing the amount of substrate in each reaction. H4 was used as a positive control. (E) Tadalafil-mediated inhibition of the arginine methylation of ALKBH7 by PRMT5. MDA-MB-231 cells with or without PRMT5 overexpression were treated with 100 μM tadalafil for 24 h. Then, cell lysates were immunoprecipitated with an anti-MYC antibody and detected with anti-SDMA and anti-ALKBH7 antibodies. (F) Western blot analysis of ALKBH7 expression in MDA-MB-231 cells treated with tadalafil (100 μM) for 24 h and CHX (100 μg/mL) as indicated before harvest.