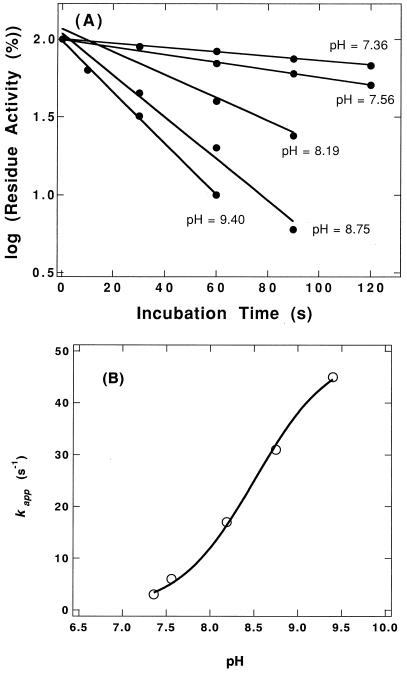
FIG. 4.
pH dependence of DTNB inactivation. (A) The enzyme solution (1 mg/ml) was incubated with DTNB (0.5 mM) at 30°C in 50 mM sodium phosphate buffer (pH 6 to 8), and 50 mM NaH2PO4-Na2B4O7 buffer (pH 8 to 9.5). The reaction was terminated at the indicated times by transferring an aliquot of the mixture to an equal volume of 50 mM phosphate buffer (pH 7.5) containing 40 mM Cys. The residue activity of the modified enzyme was measured with Cbz-G-F as the substrate at 85°C in 50 mM sodium phosphate buffer (pH 7.5) containing 5% DMF. (B) Replot of pseudo-first-order rate constant versus pH. The solid line represents the fit of the data to equation 1 with a pK of 8.51 and a kappmax of 0.050 s−1.