Figure 4.
Transplantation of 6F-ADRCs into acute myocardial infarcted tissues improves chronic cardiac function in vivo
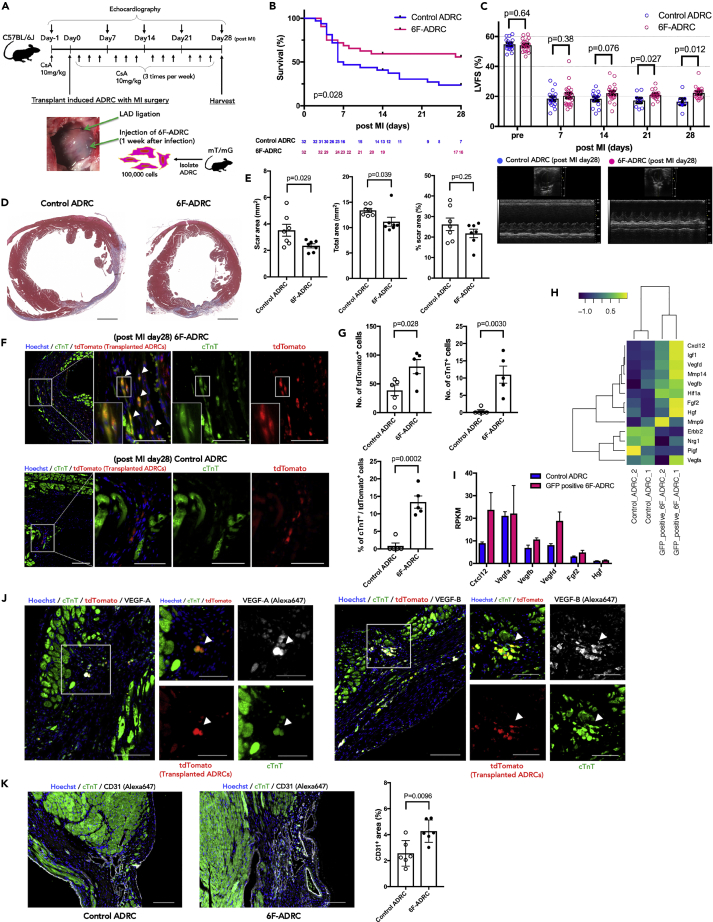
(A) Experimental scheme using 8-week-old C57Bl/6J mice subjected to intracardiac injection of 6F-ADRCs. ADRCs were harvested from mT/mG mice so that the implanted ADRCs could be identified. As the treatment group, 6F-ADRCs 1 week after viral induction were injected (6F-ADRC group). As the control group, ADRCs without viral induction were injected (Control ADRC group).
(B) Survival plots up to 28 days after coronary artery ligation followed by the implantation of ADRCs (n = 22–32). Survival curves were analyzed using Kaplan–Meier estimators and log-rank (Mantel-Cox) tests.
(C) Echocardiogram analysis of left ventricular fractional shortening.
(D) Representative images of Masson’s trichrome staining were captured 4 weeks after transplantation. Scale bars, 1,000 μm. (E) Transplantation of 6F-ADRCs decreases the scar area (left) and cross-sectional area (middle) of the heart after MI. Relatively, the percent scar area was not significantly different (right).
(F) Transplanted ADRCs labeled with tdTomato (enhanced with immunostaining of anti-RFP/Alexa 647) from mT/mG mice were identified in the border area 4 weeks after acute MI. Immunostaining showed that double-positive cells of tdTomato and cTnT (with Alexa 488) were frequently observed in the 6F-ADRCs group compared with the uninduced ADRCs group. Scale bars, 100 μm (low magnified images) and 50 μm (high magnified images) (G) The number of dtTomato-positive cells representing implanted ADRCs significantly increased in the 6F-ADRC group compared with the Control ADRC group (up right). Almost no cTnT-positive implanted cell was observed in Control ADRC group (up left); however, 13.36 ± 1.754% of implanted cells were positive for cTnT in the 6F-ADRC group (bottom) (n = 5).
(H) Heatmap image of RNA-seq analysis illustrating 13 genes related to angiogenic paracrine factors among uninduced control ADRCs and GFP+ 6F-ADRCs. Log10(RPKM+1) values obtained from RNA-seq are shown (n = 2).
(I) RNA-seq-based angiogenic paracrine signals including VEGF-A and VEGF-B expression levels of GFP+ 6F-ADRCs relative to uninduced ADRCs. RPKM values obtained from RNA-seq are shown (n = 2).
(J) Transplanted 6F-ADRCs labeled with tdTomato and stained with cTnT were stained with VEGF-A and VEGF-B in the border area 4 weeks after acute MI surgery. The left row shows VEGF-A and the right row shows VEGF-B staining. Scale bars, 100 μm (low magnified images) and 50 μm (high magnified images). (K) CD31 immunofluorescence staining of section border zone of acute MI scar area, and CD31+ fraction area, compared with Control ADRCs treatment group and 6F-ADRCs treatment group (n = 6). Scale bars, 100 μm.
Data are represented as mean ± SEM (C, E, G, I, K). Statistical significance was determined with the Student’s t-test between two groups (C, E, G, K).