Graphical abstract
Keywords: Antibiotic resistance, Biofilms, Cancer therapy, Iron overload, Siderophores, Trojan horse
Highlights
-
•
Microbial iron chelators as a new route to develop inspiring antimicrobials.
-
•
Siderophore-mimicking antibiotics as a pathogen-targeted strategy.
-
•
Effectiveness of iron chelators on antibiotic-resistant Gram-negative bacteria.
-
•
Iron chelators and the treatment of iron overload diseases.
-
•
Iron chelators as powerful tools for cancer therapy.
Abstract
Background
Bacterial infections involving multidrug-resistant Gram-negative bacteria have become critically involved in the current antibiotic crisis. This, together with the bacterial evolution ability, prioritizes the discovery of new antibiotics. Research on microbial iron acquisition pathways and metabolites, particularly siderophores, has highlighted hopeful aspects for the design of advanced antimicrobial approaches. Moreover, exploiting siderophores machinery to treat diseases associated with iron overload and cancer is of additional interest for the therapeutic arena.
Aim of Review
This review highlights and provides a renewed perspective on the evolutionary path of siderophores, from primordial siderophores to new iron chelating agents, stimulating the field to build on the past and shape the future.
Key Scientific Concepts of Review
The effectiveness of siderophore-mimicking antibiotics appears to be high and selective for Gram-negative pathogens, rendering multidrug-resistant (MDR) bacteria susceptible to killing. Herein, cefiderocol, a new siderophore antibiotic, is well positioned in the clinic to treat MDR infections instigated by Gram-negative bacteria, particularly urinary tract infections and pneumonia. This siderophore has a mode of action based on a “Trojan horse” strategy, using the iron uptake systems for efficient bacterial penetration and killing. Recent progress has also been achieved concerning new iron chelating compounds to treat diseases associated with iron overload and cancer. Though these compounds still face great challenges for a clinical application, their promising results open up new doors for the design and development of innovative iron chelating compounds, taking benefit from the structurally diverse nature of siderophores.
Introduction
Biomedical and pharmaceutical areas are facing growing challenges with the continued upsurge of multidrug resistance among bacteria, which has contributed to the global increase of infections caused by such resistant microorganisms. These bacterial infections, typically biofilm-related, are an escalating health problem, leading to a substantial rise in mortality, morbidity, and treatment costs [1], [2]. Bacterial biofilms have been associated with many chronic and recurrent bacterial infections, with up to 80% of human infections involving biofilm formation [1], [3]. Biofilm development is a complex process, multifaceted and dynamic, involving numerous mechanisms such as extracellular matrix (ECM) production, quorum sensing (QS), and nutrient and chemical signal response, with the colonizer cells inherently resistant to both host innate immune defenses and antibiotic treatments [4]. Multiple factors have been recognized to confer the multi-factorial resistance of biofilms to antibiotics. These comprise a limited diffusion of antibiotics through the biofilm ECM, reduced metabolic and growth rates, the presence of persister cells, and an altered physiology of bacteria in biofilms comparatively to the same cells in planktonic state [5], [6], [7]. Hence, bacterial biofilm formation along with antibiotic resistance has contributed to an escalating and intractable problem in the health sector.
Owing to the increasing antibiotic resistance, the focus of current research is to discover new antibiotics to address and fight multidrug-resistant (MDR) pathogens, especially Gram-negative bacteria - for which the situation is particularly serious [8]. One viable and promising strategy for the design of new antimicrobial compounds is by using or targeting bacterial virulence factors, where siderophores are included. This approach will allow escaping the selective pressure for resistance as occurs from antibiotic use. Besides that, these bacterial virulence factors affect other virulence mechanisms, in particular biofilm formation [9], [10]. Moreover, a reduced impact in the host commensal microbiome is expected from targeting these bacterial virulence factors [10].
Iron is an essential nutrient for both humans and bacteria [11]. Despite its multifaceted biological functions in humans (i.e. DNA biosynthesis, oxygen transport, cell respiration, and gene regulation), iron can be harmful at high levels because of its toxicity and ability to cause oxidative stress [12], [13]. Hence, the bioavailability of iron in mammalian hosts is strictly controlled throughout its absorption, transport, and storage. However, the iron availability is limited under aerobic conditions since the main soluble ferrous iron (Fe2+) is oxidized to its insoluble ferric form (Fe3+), being further polymerized to ferric (oxy)hydroxide [13], [14]. Furthermore, the majority of iron existing in circulation is tightly bound to host proteins like transferrin, limiting iron access to invading pathogens, which also require iron [15]. Responding to this challenge in a pathogenic context, bacteria synthesize and secrete low molecular weight molecules known as siderophores, which can acquire and solubilize iron from the host [16], [17]. Siderophores are natural iron chelators and their biosynthesis is driven by the iron concentration in the surrounding environment. These iron chelators are secreted out for iron acquisition when the bacteria detect limited iron levels, with further scavenging and binding of iron to form an iron-siderophore complex that is recognized and translocated inside the cells by specific cell-surface receptors [16], [17]. Both Gram-positive and Gram-negative produce siderophores (Table 1), however, their iron uptake mechanisms are different.
Table 1.
Common siderophores produced by bacteria and the respective chemical group.
| Group | Siderophores | Bacteria | References |
|---|---|---|---|
Catecholate
|
Bacillibactin |
Bacillus cereus Bacillus subtilis |
[18], [19], [20] |
| Enterobactin |
Escherichia coli Salmonella spp. Klebsiella pneumoniae |
||
| Salmochelin |
Salmonella spp. Klebsiella pneumoniae |
||
| Agrobactin |
Agrobacterium spp. Rhizobium radiobacter |
||
| Vanchrobactin | Vibrio anguillarum | ||
| Anguibactin | |||
Hydroxamate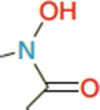
|
Aerobactin |
Shigella flexneri Aerobacter aerogenes Escherichia coli Klebsiella pneumoniae |
[21], [22], [23], [24] |
| Alcaligin |
Alcaligenes eutrophus Bordetella bronchiseptica |
||
| Ferrioxamine B | Salmonella enterica Yersinia enterocolitica |
||
Carboxylate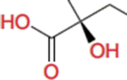
|
Rhizobactin |
Rhizobium meliloti Sinorhizobium meliloti |
[19], [25], [26] |
| Staphyloferrin | Staphylococcus aureus | ||
Phenolate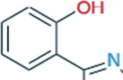
|
Pyochelin | Pseudomonas spp. | [27], [28] |
| Mixed | Pyoverdine | Pseudomonas spp. | [19], [28], [29] |
| Ferrichrome | Yersinia enterocolitica Pseudomonas putida |
||
| Yersiniabactin | Yersinia enterocolitica Klebsiella pneumoniae |
In Gram-positive bacterial pathogens, the uptake of iron-siderophore complexes comprises an ATP-binding cassette (ABC) transporter and a membrane-anchored binding protein [30], [31]. In Gram-negative bacteria, active transport of these complexes requires a specific outer membrane receptor, namely TonB system (TonB, ExbB, ExbD), an inner membrane ABC transporter and a periplasmic binding protein [30], [31]. The large structural and functional diversity of siderophores, commonly divided into catecholate, hydroxamate, carboxylate and phenolate–according to the moieties involved in iron chelation (Table 1), constitute a valuable chemical library for the design of specific siderophore-mimicking antibiotics [16], [32]. In addition, the involvement of siderophores at the root of numerous bacterial processes makes these therapeutic antimicrobial compounds even more attractive [33], [34]. Extensive research on this topic has revealed the identification and approval of a new siderophore cephalosporin antibiotic – cefiderocol – with powerful antibacterial action on MDR Gram-negative pathogens [35], [36]. Such siderophore-based compounds could potentially give a boost in dealing with biofilm infections and, consequently, contribute to circumvent the antibiotic resistance crises.
It has become clear that siderophores have biological properties that extend beyond simple iron acquisition. Iron overload is known to be a common complication for the treatment of many diseases like sickle cell disease and thalassemia, which are among the most frequent monogenic global disorders [37], [38]. The reduction of body iron overload to normal range levels using siderophores as an effective chelation therapy is promising to decrease the morbidity and mortality rates from these disorders. In fact, the iron chelation ability, from primordial siderophores to new designed iron chelating agents, has already been translated for clinical use to treat iron overload diseases [39], [40], [41]. Moreover, the involvement of iron chelating agents in cancer therapy has also been increasingly evidenced [42], [43]. Iron excess may lead to an increased risk for developing cancer, and siderophores can contribute to iron homeostasis [44], [45].
Given the involvement and importance of siderophores for both physiology and pathogenicity of bacteria, using or targeting such a bacterial pathway seems to be a deep well for developing new antimicrobial agents. Moreover, exploiting siderophores machinery for the treatment of many other diseases such as iron overload diseases and cancer, gives future directions to the therapeutic arena [46], [47], [48]. Despite a large number of original research on the topic, no recent attempt has been made to review and critically address the progress on the mechanisms involving siderophores in biofilms, cell biology and survival, and their consequent use as therapeutics. This review seals this gap and discusses the evolutionary path of siderophores, from primordial siderophores to new iron chelating agents, with a critical emphasis on in vitro, in vivo, and available clinical information. The review starts with a brief overview of siderophores biology along with an in-depth analysis of their mechanism of action, followed by recent findings on their exploitation in the clinical context, examining their potential as new antimicrobial compounds and iron chelators to treat diseases associated with iron overload and cancer. The most efficient compounds that have reached clinical trials are highlighted. This study is not envisioned to be an exhaustive comprehensive review of the literature on siderophores, but an investigation of the progress in their development for antimicrobial therapy, iron overload diseases and cancer, from 2000 to 2021.
Leveraging bacterial biofilm mechanisms to develop antimicrobials and iron chelating agents
Understanding bacterial biofilm mechanisms is fundamental to develop effective control strategies. Biofilm development depends on the synthesis of specific molecules. The comprehensive knowledge of the specific pathways involved can provide insights on new therapeutic targets for drug discovery. The biofilm formation process typically encompasses cell–cell interaction mechanisms, involving both regulatory mechanisms and the synthesis of secondary metabolites, including siderophores (Fig. 1) [49].
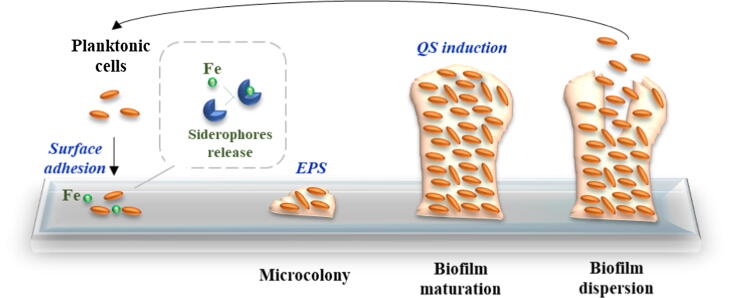
Fig. 1.
Bacterial biofilm formation and development. During the complex process for biofilm formation, bacteria secret secondary metabolites, namely siderophores, to chelate iron (Fe) essential as a signal for biofilm development. Then, bacteria adhere to surfaces by adsorption and form a microcolony through the secretion of extracellular polymeric substances (EPS). In later stages, the biofilm is mature and densely populated due to the induction of the quorum sensing (QS) responsible for the regulation of genes involved in biofilm maturation and maintenance. Lastly, some bacteria start to detach and the biofilm disperses (adapted from Landini et al. [50] and Post et al. [10]).
One of the main regulatory mechanisms in biofilms is recognized as quorum sensing (QS). QS is a communication mechanism between bacteria by releasing, sensing and responding to small diffusible signal molecules. Indeed, several bacteria are able of using QS mechanisms to regulate biofilm formation [51]. QS induction is also involved in biofilm maturation and dispersion (Fig. 1) [50]. Moreover, pathogenic bacteria in biofilms use QS mechanisms to trigger virulence and develop resistance to antibiotics. In addition to this regulatory mechanism, bacterial secondary metabolites, particularly siderophores, also possess a significant role in several cellular processes in biofilms, being likewise responsible for virulence and infection [9], [52], [53]. These chelating agents, together with iron, are essential for the switch between planktonic to sessile state, including the gene expression in biofilms [9], [52], [53], [54]. Singh et al. [52] demonstrated that lactoferrin (a human iron transport protein), used to chelate free iron, delayed Pseudomonas aeruginosa biofilm formation under concentrations needed to inhibit planktonic growth. Moreover, iron chelation by lactoferrin prevented the planktonic cells from attaching to surfaces, the first crucial stage involved in biofilm development (Fig. 1). Several other eye-catching works showed the ability of iron chelators to disrupt and/or kill mature biofilms [52], [53], [54]. The regulation of biofilm formation by iron with siderophore-dependent pathways (Fig. 2) has been largely demonstrated in many bacterial species [55], [56], [57]. For instance, Modarresi et al. [33] observed the role of iron in the siderophore-producing bacterium Acinetobacter baumannii, an opportunistic pathogen responsible for causing a wide variety of diseases ranging from urinary tract infections to more serious conditions like ventilator-associated pneumonia and sepsis [34]. They found that QS and biofilm formation were regulated by iron concentration in a dose dependent manner, indicating that iron limitation plays a fundamental role in siderophore production that results in strong or weak biofilm production [34]. Wu and Outten [58] evaluated the role of iron availability in regulating biofilm formation by E. coli and observed that biofilm formation was repressed under low iron conditions. Banin et al. [55] showed the importance of iron in biofilm formation by P. aeruginosa, an opportunistic bacterium involved in various infections, ranging from septicemia, urinary infections, and cystic fibrosis [56]. It was observed that P. aeruginosa mutants, which cannot acquire iron via the iron acquisition system, formed thin biofilms similar to those developed by the wild-type in low iron conditions [55]. Nevertheless, when an excess of iron was provided to the mutant, similar biofilm development to wild-type strain occurred [55]. Additionally, Chen et al. [57]found a slower growth of K. pneumoniae in iron restricted environments. K. pneumoniae is the most clinically relevant species of Enterobacteriaceae and is known to cause both community-acquired and nosocomial infections. Moreover, iron promoted K. pneumoniae biofilm formation, while the presence of an iron chelator attenuated biofilm formation. Collectively, these data reveal that iron plays a critical role in the bacterial biofilm formation process.
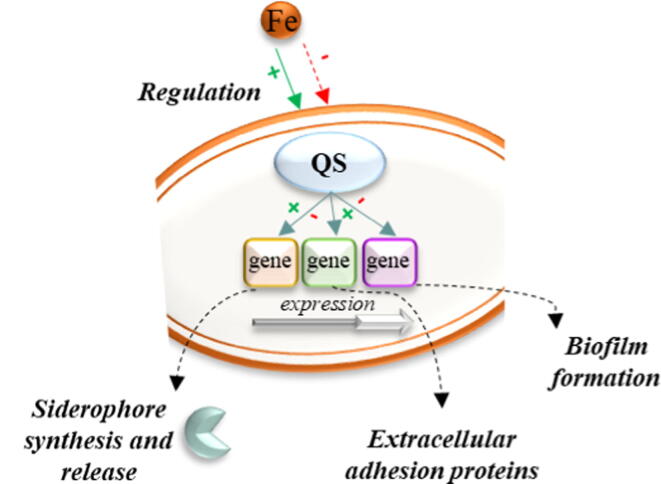
Fig. 2.
Role of iron in acquisition mechanisms and bacterial biofilm formation. The presence of iron (Fe) can positively or negatively regulate quorum sensing (QS) regulators. A specific iron concentration is necessary for biofilm formation. If not, the presence of iron can inhibit the expression of genes responsible for extracellular protein synthesis, through QS regulators, necessary for bacterial surface adhesion. In addition, biofilm formation can be directly regulated by QS systems.
The relationship between iron, siderophore biosynthesis and iron chelation in biofilm formation is evident. This provides a better understanding of the iron signaling cascade critical for biofilm development, which could help in the rational design of innovative therapeutic agents. Furthermore, benefiting from the advances and findings of the biological function and trafficking of siderophores, there has been a paradigm shift toward potential vulnerabilities of these molecules that can be exploited clinically, especially for the antimicrobial field and for the treatment of diseases associated with iron overload and cancer.
Siderophores for antimicrobial therapy
New antimicrobial compounds based on siderophore-based agents and/or siderophore-targeting are an auspicious path against MDR bacteria, which could help clinicians fight against antibiotic resistant pathogens. The development of new antimicrobial drugs faces specific challenges on MDR Gram-negative bacterial pathogens, mostly on P. aeruginosa, A. baumannii, and Enterobacteriaceae, categorized by the World Health Organization (WHO) as crucial bacteria that cause the greatest threat to human health [8]. However, the progress translating the Gram-negative bacteria clinical pipeline has been slow, partly owing to the difficulty overcoming the outer lipid membrane and associated efflux pumps in these bacteria, which have become resistant to carbapenems and the third generation of cephalosporins [8], [59]. One approach for circumventing the resistance displayed by Gram-negative pathogens involves exploiting the iron uptake path of these bacteria through the conjugation of a siderophore to an antibiotic. This promotes the entry of the compound into cells when iron acquisition processes are expressed [28], [60]. In line with this, several siderophore-antibiotic conjugates were already designed and tested. The results demonstrated good in vitro action on several clinically relevant MDR Gram-negative bacteria [61], [62], [63]. However, further development of candidates has not reached the market because of resistance mechanisms, dearth of reliable in vivo effectiveness and occurrence of side effects [61], [62], [64]. For instance, the siderophores monocarbam SMC-3176 (Fig. 3A) and monobactam MB-1 (Fig. 3B) have not advanced into clinical studies due to the lack of correlation between good in vitro data and in vivo responses in P. aeruginosa [61], [62]. These compounds carried a risk of reduced efficiency in P. aeruginosa because of the quick adaptative resistance [61], [62]. Moreover, in a work that compared the in vivo efficacy of SMC-3176, MB-1 and a new siderophore cephalosporin, known as cefiderocol (Fig. 4A), against P. aeruginosa strains, the attenuated in vivo efficiencies of the siderophores SMC-3176 and MB-1 were verified, while cefiderocol exhibited a powerful effect in vivo on all P. aeruginosa strains tested, including the resistant ones [65].
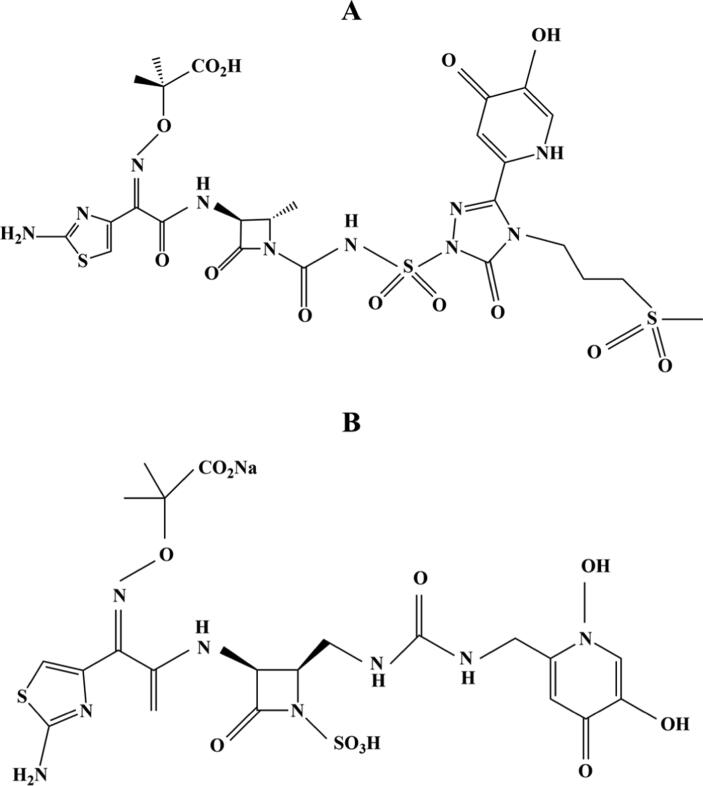
Fig. 3.
Chemical structures of SMC-3176 (A) and MB-1 (B).
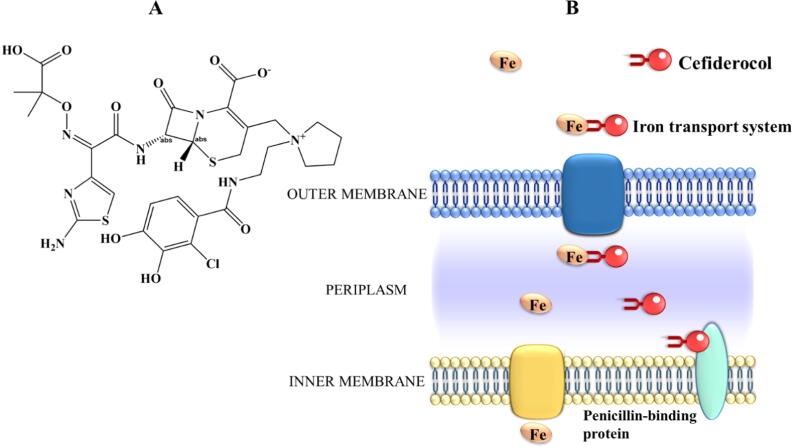
Fig. 4.
Chemical structure of cefiderocol (A) and its “Trojan horse” active transport mechanism to across the outer membrane of Gram-negative bacteria using the iron transport system (B). Then, cefiderocol is released into the periplasmic space, attaches to penicillin-binding proteins and inhibits the bacterial cell wall synthesis, causing cell death.
The antimicrobial efficacy of this advanced-generation cephalosporin has been assessed using several pathogenic bacteria exhibiting potent and broad in vitro and in vivo action against carbapenem-resistant strains of Enterobacteriaceae [66], [67], A. baumannii [67], [68], P. aeruginosa [67], [68], [69], [70], and K. pneumoniae [68], [70]. Several animal models have been used to demonstrate the promising in vivo efficacy of cefiderocol, including murine lung infection models [68], [71], neutropenic murine thigh infection models [67], [69], [71], and murine urinary tract infection models [70]. Based on these and other in vivo data, the clinical efficacy of cefiderocol has been assessed, and it was the first cephalosporin antibiotic to further advance the phase 1 human clinical assays to reach a stage of clinical development. This new siderophore (earlier identified as S-649266) employs a “Trojan horse” transport mechanism that allows entry to Gram-negative bacterial pathogens by exploiting the bacterial iron-siderophore uptake system (Fig. 4B) [66], [72]. Cefiderocol has a catechol moiety on the 3-position of the R2 side chain attached to the cephalosporin molecule, which chelates free iron [73], [74]. Then, this complex is transported via the bacterial iron transport system across the outer membrane of Gram-negative bacterial pathogens into the periplasmic space, attaches to penicillin-binding proteins and inhibits the bacterial cell wall synthesis, causing cell death [73], [75]. In Portsmouth et al. [76], a phase 2, randomized, double-blind, non-inferiority phase 2 clinical study was performed to evaluate the efficacy and safety of cefiderocol, compared to imipenem-cilastatin, for the treatment of complicated urinary tract infections (cUTI) instigated by Gram-negative uropathogens (NCT02321800). Though envisioned as a non-inferiority assay, the outcomes of this clinical trial favoured cefiderocol (see Table S1 A in Supplementary Information for study details). Its superiority for the primary endpoint was boosted by the higher eradication of Gram-negative bacteria at test of cure (73% vs 56% with imipenem-cilastatin), since the clinical outcome was equivalent for both groups (90% cefiderocol vs 87% imipenem-cilastatin). The higher number of patients that presented a microbiological outcome in the cefiderocol group was suggested to be related to improved drug penetration into biofilms, a key factor associated with recurrent UTIs, in parallel with the distinctive bactericidal mode of action of cefiderocol. Furthermore, the safety profile of cefiderocol was demonstrated. Based on this clinical trial, the Food and Drug Administration (FDA) recently approved cefiderocol (Fetroja®, November 2019) for the treatment of 18 years old patients or older, with cUTI, including pyelonephritis caused by Gram-negative pathogens [35].
Very recently, FDA authorized the approval of a supplemental New Drug Application (sNDA) for cefiderocol (Fetroja®, September 2020) for the treatment of 18 years old patients or older, with hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) caused by Gram-negative bacteria resistant to other antibiotics [35]. This consent was focused on the outcomes of a randomized, double-blind, phase 3, non-inferiority clinical trial that compared the efficacy and safety of cefiderocol with high-dose, extended-infusion meropenem in patients with HAP, VAP, or healthcare-associated pneumonia (HACP) (NCT03032380) [77]. That study showed the non-inferiority of cefiderocol to high-dose, extended-infusion meropenem (12.4% vs 11.6%, respectively), infused over 3 h, in patients with nosocomial pneumonia involving a variety of Gram-negative pathogens, particularly P. aeruginosa, A. baumannii and Enterobacterales (see Table S1 B in SI for study details). This primary goal was then supplemented by microbiological and clinical secondary responses. The microbiological eradication at test of cure in the modified ITT population was 48% in both groups, whereas the clinical outcome was achieved by 65% vs 67% of patients in the cefiderocol and high-dose meropenem group, respectively. In addition, the safety profile of cefiderocol was observed, which is in agreement with the safety findings obtained in other studies of cefiderocol, including that of Portsmouth et al. [76]. That work was carried out in a population of critically ill patients at high-risk, representative of the present aetiology and epidemiology of nosocomial pneumonia. Furthermore, these clinical data highlighted the extensive coverage of cefiderocol against all Gram-negative bacterial pathogens considered of critical priority by the WHO [8].
Currently, three clinical studies in phase 2 for the treatment of adult patients with bloodstream infections (NCT03869437), hospitalized pediatric patients with Gram-negative bacterial infections (3 months to < 12 years) and cUTI (3 months to < 18 years) (NCT04215991), and hospitalized pediatric patients with Gram-negative bacterial infections (3 months to < 18 years) (NCT04335539) are ongoing.
Siderophores and new iron chelating agents for the treatment of diseases associated with iron overload and cancer
Siderophores have proven to be powerful iron chelating agents for the clinical treatment of diseases with iron overload like sickle cell disorder and thalassemia, and cancer. Thalassemia and sickle cell disease are among the most frequent monogenic global disorders [78], [79]. Thalassemia is a result of mutations in globin genes that cause the reduction or absence of hemoglobin synthesis, presenting defects in the synthesis of either β-like (β-thalassemia) or α-like (α-thalassemia) globin chains [78], [80]. On the other hand, sickle cell disease is a result of a homozygous missense mutation of the β-globin gene that triggers polymerization of hemoglobin S, and it is characterized by unpredictable episodes of acute illness and progressive organ injury [79], [81]. Due to their impact on morbidity and mortality, these disorders are increasingly being recognized as a global health problem. Blood transfusion is the mainstay standard therapy for the control and treatment of thalassemia and sickle cell disease [82]. However, many of these patients require repeated transfusions, which causes significant iron overload [82]. This overloading can lead to iron deposition in vital organs such as brain, heart, liver and endocrine glands [83]. The major cause associated with this organ injury is the overproduction of ROS in the presence of excess iron [83]. As the human body has no active excretion mechanisms for excess iron, new agents based on iron chelators have been under development and approval [84], [85]. Chelation therapy aims to stimulate the iron excretion in patients having iron overload and to maintain or return body iron to safe levels [84].
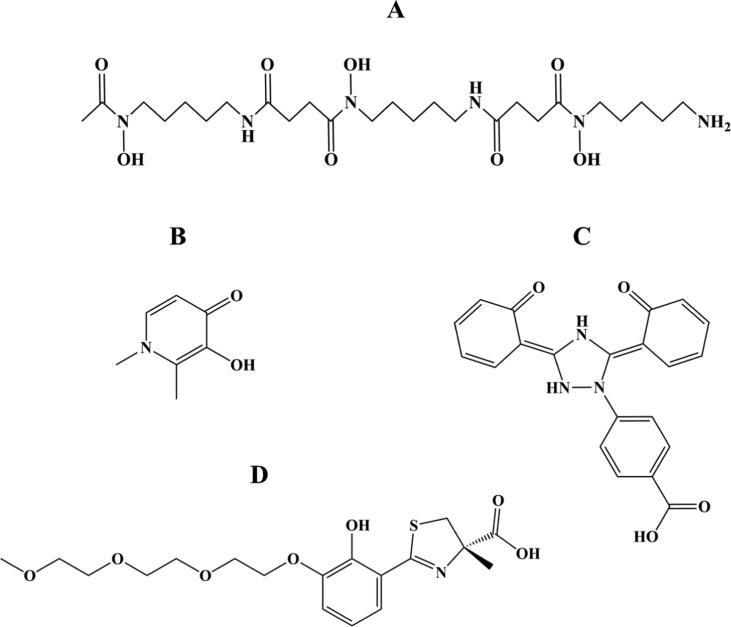
Various randomized clinical trials have been performed to assess the efficacy and safety of iron chelation implicated in the treatment of iron overload [37], [38], [86], [87], [88]. Deferoxamine (Desferal®, DFO), deferasirox (Exjade®, DFX), and deferiprone (Ferriprox®, DFP) have been the most important US FDA-approved iron chelators over the past years (Fig. 5) [89]. Deferoxamine (Fig. 5A), a hexadentate chelator binding iron at 1:1 M ratio, was the first iron chelator introduced into clinical practice [90]. Despite the great iron-scavenging features of deferoxamine, the short plasma half-life (20–30 min) and poor oral bioavailability of this iron chelator, based on a subcutaneous administration over 8–12 h and 5–7 days/week, results in poor compliance [82], [90], [91]. Responding to this demanding therapeutic regimen, two orally active iron-chelating compounds known as deferasirox and deferiprone have received approval for the treatment of iron overload. Deferiprone (Fig. 5B) is a small molecule that binds to iron in a 3:1 ratio and presents a relatively short half-life (3–4 h, three times daily), while deferasirox (Fig. 5C) binds to iron in a 2:1 ratio and have long half-life ranging from 8 to 16 h, which can be given once daily, providing a 24-hours chelation coverage [82], [91], [92]. Combined chelation therapy has also been introduced as a means to manage iron overload when therapy based on a single chelating compound is not effective. Several studies have demonstrated the efficacy and safety of using combined chelation treatment to remove iron overload, with different combinations being tested in clinical practice, including with deferoxamine/deferasirox [93], [94], deferoxamine/deferiprone [95], [96], and deferiprone/deferasirox [97], [98]. This combined or alternated chelation treatment has been shown to decrease systemic and myocardial iron, provide excellent control of the toxic label plasma iron species without increasing the toxicity, and improve the endothelial and ventricular function in thalassemia patients presenting mild to moderate cardiac iron loading [93], [95], [98].
Fig. 5.
Chemical structures of deferoxamine (A), deferiprone (B), deferasirox (C), three of the most important US FDA-approved iron chelating compounds implicated in the treatment of iron overload, and FBS0701 (D).
Notably, based on all these findings, iron chelators bring significant hopeful implications for the patients safety and life expectancy. For instance, without iron chelation therapy, the mean survival from birth in thalassemia major patients were 12–17 years, with death occurring mostly due to cardiac failure or arrhythmia [99]. Although each of these approved agents, applied alone or in combination, has been shown to effectively sequester excess iron, several parameters such as the severity of iron overload, the clinical situation of the patient, treatment period, and respective final costs must be taken into reflection when choosing the proper chelation therapy for a specific clinical case [89]. So far, all these iron chelators appear to be effective and well-tolerated, however, their poor compliance (deferoxamine) [82], [90], [91] and adverse effect profiles such as gastrointestinal symptoms (deferiprone and deferasirox) [86], [88], [90], [100], agranulocytosis (deferiprone) [86], [100] and renal damage (deferasirox) [39], [101], have pushed researchers to identify new oral iron chelators.
New oral chelators have reached the phase of clinical development such as FBS0701, also known as SPD602, SSP-004184 or SSP-004184AQ (Fig. 5D) [102], [103]. The oral iron chelator FBS0701 is included in the desazadesferrithiocin class of siderophore-related tridentate chelators [103]. In phase 1 clinical investigation, the multidose safety and pharmacokinetics studies established the safety of FBS0701, with a mean half-life of 16.2–21.3 h, suggesting the feasibility of once-daily dosing in iron-overloaded patients [102]. A phase 2, randomized, multicenter, clinical trial, designed to assess the efficacy, safety, and pharmacodynamics of FBS0701 in the treatment of chronic iron overload, demonstrated its good capability in iron chelation, presenting a similar profile to currently approved iron chelating compounds (NCT01186419) [103]. Besides the occurrence of adverse effects, these did not appear to be dose related and happened at low frequency [103]. Nevertheless, three clinical studies conducted in phase 2 (NCT01363908; NCT01604941; NCT01671111) were terminated because of the interruption in treatment with FBS0701 and the inability at the time to draw definitive conclusions from the data.
Interestingly, the iron chelator FBS0701 has also shown potential for the treatment of malaria, one of the most prevalent and deadly parasitic diseases worldwide [104]. The clinical indication of drug resistance to the existing antimalarial agents as well as the spread of resistant parasite strains further increases the interest in iron chelating therapy [104]. For instance, in a work developed by Ferrer et al. [105] the iron chelator FBS0701 was found to exhibit antimalarial properties against Plasmodium blood-stage infections in vitro and in vivo. FBS0701 demonstrated a single oral dose cure of the lethal Plasmodium yoelii murine malaria model and this result was observed to persist after the chelator has cleared from plasma [105]. This iron chelator can be administered as a single daily dose due to its propitious adsorption and pharmacokinetic properties compared to deferoxamine and deferiprone [102]. The ability of FBS0701 to remove labile iron from erythrocytes was likely primarily responsible for both the antimalarial activity and the prolonged effect of this chelator [105]. In another work of Ferrer et al. [105] the effect of FBS0701 on stage V gametocytes infectivity to mosquitos was evaluated and it was observed a substantial dose related reduction in mosquito infectivity. This decline on mosquito infectivity was demonstrated to be a result of iron chelation, as pre-incubation of FBS0701 with ferric chloride reduced the inhibitory effect and restored gametocyte infectivity. Thus, FBS0701 offers an interesting alternative or complementary approach to current therapeutic agents for the treatment of malaria.
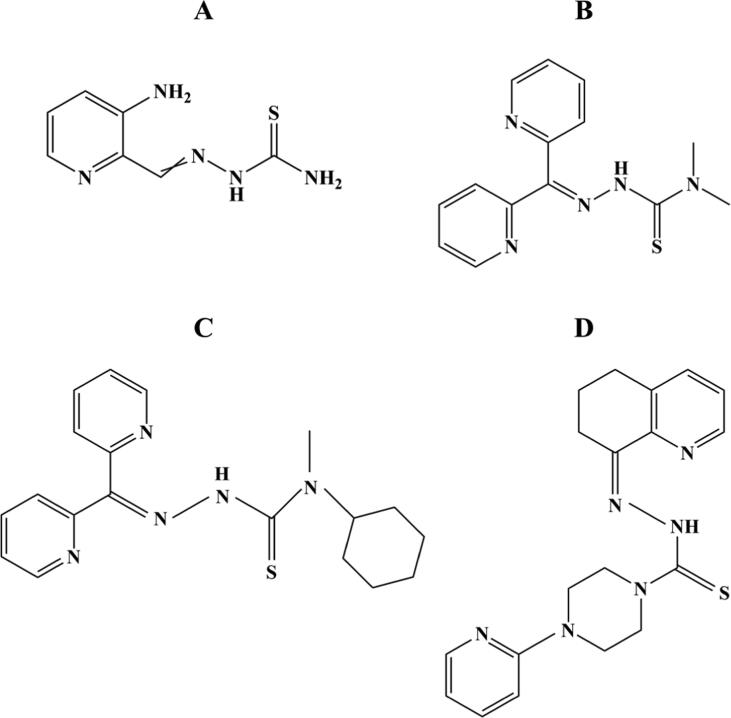
Iron has also been implicated in playing major functions in cancer development, proliferation, and metastasis [106], [107]. Cancer occurrence and mortality is increasing rapidly globally. Indeed, cancer has emerged as the first or second prominent reason for death before age 70 years in 91 of 172 countries and third or fourth ranking in 22 more countries, by a WHO estimate [108]. The malign cancer phenotype is often associated with dysregulated iron homeostasis, as its excess may lead to an increased risk of developing cancer [106], [107]. This iron overload plays a vital role in cancer advancement, either by promotion of tumor development, cell proliferation, and metastatic cascade or by involvement in redox reactions responsible to catalyze the generation of ROS and boost oxidative stress [107], [109]. Hence, iron homeostasis modulations such as iron depletion through chelators make it a possible therapeutic target for cancer. Some iron chelators have already been put into clinical assessment, including deferoxamine (Fig. 5A), and triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) (Fig. 6A) [110].
Fig. 6.
Chemical structures of triapine (A), Dp44mT (B), DpC (C) and COTI-2 (D), four iron chelating agents designed and evaluated for cancer.
Deferoxamine, a siderophore currently employed in the clinical treatment of diseases associated with iron overload, was the first iron chelator to be assessed for its anticancer properties. While deferoxamine showed some useful anticancer activity, its clinical efficacy has been limited, which relates to the fact that it was not developed specifically for cancer therapy [111]. Nevertheless, the promise of deferoxamine as anticancer agent has prompted the design of more effective iron chelators, with a particular focus on triapine.
Triapine is a tridentate chelator that has been assessed in phase 1, 2 and 3 clinical studies for cancer therapy. This iron chelator has been assessed either as a single agent or combined with traditional chemotherapy and/or radiation therapy in multiple phase 1 and phase 2 studies for different types of cancer (see Tables S2 and S3, SI). Triapine is a strong inhibitor of ribonucleotide reductase (RR), an enzyme implicated in the reduction of the four ribonucleotides to their related deoxyribonucleotides needed for DNA synthesis and repair [112], [113]. Increased RR activity has been related to tumor cell formation and metastasis [114]. By inhibiting the RR, DNA synthesis and cell proliferation are disrupted, causing cell death [112], [113]. Therefore, this enzyme has long been believed to be a key target for cancer therapy. Early clinical data demonstrated that the use of triapine as monotherapy may not generate survival advantages in patients with cancer [115], [116], [117].
Nevertheless, when triapine was combined with additional chemotherapeutic compounds great potential in controlling certain cancers was observed [44], [45], [118], [119]. Interestingly, a phase 1 clinical trial developed by Kunos et al. [45] showed that triapine was well-tolerated at a three times weekly (25 mg/m2 dose) in combination with cisplatin and pelvic radiation for the treatment of advanced cervical cancer (see Table S4 A in SI for study details). All patients presenting stage IB2 to IVB cervical cancer reached complete clinical outcomes and persisted without disease relapse, with a median of 18 months of follow-up (6–32 months). In line with this data, a phase 2 clinical trial of daily pelvic radiation and once-weekly cisplatin (40 mg/m2) plus three times weekly triapine (25 mg/m2) for patients presenting cervical and vaginal cancer was performed (NCT00941070) [119]. It was observed that the combination of triapine with cisplatin-radiotherapy enhanced the metabolic complete outcome from 69% to 92, also raising the 3-year progression-free survival estimation from 77% to 92% (see Table S4 B in SI for study details). Moreover, a favorable safety profile was observed and no significant symptomatic methemoglobinemia was reported after triapine administration. Accordingly, a very recent phase 3 clinical study of triapine-cisplatin-radiotherapy in patients with advanced-stage uterine cervix or vaginal cancers, to assess progression-free and overall survival, is being performed (NCT02466971).
Overall, all the clinical trials of triapine described above have interesting clinical prospective and the results of the more recent investigations should be of interest. Nevertheless, some off-target side effects observed in the treatment of patients with triapine such as gastrointestinal symptoms, neutropenia, myelosuppression, hypoxia, and methemoglobinemia have raised some concerns regarding its clinical use [117], [120], [121], [122]. Therefore, research has also begun to focus in designing more effective and selective iron chelators for cancer therapy. New thiosemicarbazone iron chelators with anticancer potential have been designed and synthesized, including 2-benzoylpyridine thiosemicarbazones (BpT) and di-2-pyridylketone thiosemicarbazones (DpT). One of the best described chelators of the DpT is the di2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT) (Fig. 6B), which has demonstrated evident and selective in vitro and in vivo antiproliferative effect in different types of cancer cells [123], [124], [125]. In addition, a thiosemicarbazone of the second generation of DpT analogues, known as di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC) (Fig. 6C), has shown marked and selective antitumor activity as well as favorable pharmacological properties and safety profile [126], [127]. Importantly, despite the structural similarities between this compound and Dp44mT, DpC has a number of key advantages. It was found that DpC does not cause cardiac fibrosis, even when used at significantly high doses [126], [128], does not generate oxyhemoglobin oxidation in vivo [129], and exhibits marked in vivo activity after oral and intravenous administration [128]. Moreover, DpC demonstrated greater efficacy than Dp44mT in vivo against aggressive pancreatic tumor and neuroblastoma xenografts [126], [130].
Interestingly, an innovative small molecule that has also recently entered a clinical trial (NCT02433626) is the third-generation thiosemicarbazone known as COTI-2 (Fig. 6D), which was found via in silico computer-aided drug design in a study performed by Salim et al. [131]. COTI-2 was shown to be active against a wide diversity of human cancer cell lines with different genetic mutation backgrounds and xenografts that are usually difficult to treat. In addition, most treated cancer cells lines showed susceptibility to COTI-2 treatment at nanomolar concentrations. COTI-2 also demonstrated a favorable safety profile in mice and superior activity against cancer cells, both in vitro and in vivo, when compared to standard chemotherapy agents (cisplatin and carmustine) and targeted-therapeutic drugs (cetuximab and erlotinib) [131]. In another more recent work Vareki et al. [132] evaluated the effect of combining COTI-2 with first-line therapeutic agents carrying different modes of action as well as whether cancer cells develop acquired- and cross-resistance to COTI-2. The combination of COTI-2 with multiple chemotherapeutic and targeted drugs improved their activity in vitro and in vivo. For instance, COTI-2 when combined with paclitaxel or cisplatin enhanced the activity of these drugs in small cell lung cancer cells. Moreover, COTI-2 was found to induce substantial tumor growth inhibition when combined with paclitaxel in a human endometrial tumor model. The combination of COTI-2 with cetuximab or erlotinib also synergistically improved the efficacy of these targeted agents against human colorectal cancer cells. Importantly, as it is well-known, the emergence of resistance is a rising problem in oncology. While cancer cells demonstrated higher levels of acquired resistance to chemotherapeutic agents such as paclitaxel and cisplatin, after each round of treatment, these cells remained sensitive to COCI-2 across multiple generations. Furthermore, chemo-resistant cancer cell lines also showed no or little cross-resistance to COTI-2. Thus, these findings suggest that COTI-2 may be useful in salvage treatment after standard therapy failure as well as in combination treatment [132]. Employing iron chelators in cancer treatment remains challenging, however, to date none of them has obtained approval for clinical use.
Conclusions and future perspectives
Siderophores, extremely versatile molecules capable to chelate iron from the surrounding environment, provide a promising source for discovering new and innovative antibiotics. MDR infections caused by Gram-negative bacterial pathogens have become one of the critical reasons for the failure of clinical treatment with the existing antibiotics. The benefits presented by siderophores are evident and include improved antibacterial efficacy, increased selectivity for Gram-negative pathogens, and making MDR bacteria more prone to killing, being further represented by cefiderocol. Its distinctive structural characteristics were an asset to surpass earlier problems faced in fighting Gram-negative pathogens. Such molecules add substantial value and reinforce our current antibiotic arsenal in the clinic, namely for the treatment of urinary tract infections and pneumonia instigated by MDR Gram-negative bacteria.
The further development and use of iron chelating agents from primordial siderophores like deferoxamine to new clinically designed iron chelators, mostly for the treatment of diseases associated with iron overload and cancer, has also been watched with interest. Numerous sophisticated and versatile iron chelating agents have been developed and characterized for their effectiveness in various randomized clinical trials. However, despite the approval and significant hopeful implications of some iron chelators for the safety and life expectancy of patients with iron overload diseases, including deferoxamine, deferiprone and deferasirox, they carry some adverse effect profiles. In this sense, new iron chelators have been developed and undergoing clinical evaluation, however, without reaching the clinical practice. In the same way, although some iron chelators have been designed for cancer therapy, none of them has attained approval for clinical use. Thus, these iron chelating compounds still face great challenges, particularly associated with their translation to a clinical real-word scenario. New siderophore-based antibiotics and iron chelating compounds will likely continue to be discovered taking advantage of the structurally diverse nature of siderophores, having in mind that over 500 siderophores were already identified [133].
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Marta Ribeiro: Conceptualization, Methodology, Investigation, Writing – original draft. Cátia A. Sousa: Conceptualization, Investigation, Writing – original draft. Manuel Simões: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by: Base Funding—UIDB/00511/2020 of the Laboratory for Process Engineering, Environment, Biotechnology and Energy – LEPABE - funded by national funds through the FCT/MCTES (PIDDAC); Projects Biocide_for_Biofilm—TDC/BII-BTI/30219/2017—POCI-01-0145-FEDER-030219; ABFISH–PTDC/ASP-PES/28397/2017-POCI-01-0145-FEDER-028397, Germirrad—POCI-01-0247-FEDER-072237 funded by FEDER funds through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES; Project “HealthyWaters – Identification, Elimination, Social Awareness and Education of Water Chemical and Biological Micropollutants with Health and Environmental Implications”, with reference NORTE-01-0145-FEDER-000069, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Biographies

Marta Ribeiro is graduated in Biomedical Engineering and received her PhD in Biomedical Engineering from the Faculty of Engineering of the University of Porto (FEUP) in 2017. From 2018 to 2020, she worked as a postdoctoral researcher at the Nanomedicine Group, International Iberian Nanotechnology Laboratory (INL), Braga. Currently, she is a postdoctoral researcher at the Department of Chemistry and Biochemistry (CIQUP), Faculty of Sciences of the University of Porto (FCUP), and Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE), FEUP. Marta Ribeiro research interests lie in advanced biomaterials, including hydrogels and composites, and biofilm-related infections.

Cátia Sousa is graduated in Chemical Engineering and received her PhD in Environmental Engineering from Faculty of Engineering of University of Porto (FEUP) in 2019. She is currently a postdoctoral researcher at the Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE) in the Department of Chemical Engineering – FEUP. Since 2019, she was appointed as Invited Assistant Professor at Polytechnic Institute of Porto (Department of Chemical Engineering). Her current research focuses on bacterial cell metabolism, mixed cultures, biofilm formation, behavior and control.

Manuel Simões has a PhD in Chemical and Biological Engineering and is currently Associate Professor with Habilitation at the Faculty of Engineering of the University of Porto. He has more than 180 papers published in journals indexed in JCR (h-index = 47), 4 books (1 as author and 3 as editor) and more than 40 chapters in books. He is section Editor-in-Chief for Antibiotics, Associate Editor for Biofouling and Frontiers in Microbiology journals. He has been highlighted as a highly cited author in 2020 by Clarivate Analytics. SCOPUS ID: 55608338000; Orcid ID: 0000-0002-3355-4398.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.10.010.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Magana M., Sereti C., Ioannidis A., Mitchell C.A., Ball A.R., Magiorkinis E., et al. Options and limitations in clinical investigation of bacterial biofilms. Clin Microbiol Rev. 2018;31(3) doi: 10.1128/CMR.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenover F.C. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119(6):S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Verderosa A.D., Totsika M., Fairfull-Smith K.E. Bacterial biofilm eradication agents: a current review. Front Chem. 2019;7:824. doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostakioti M., Hadjifrangiskou M., Hultgren S.J. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a010306. a010306–a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 6.Grassi L., Maisetta G., Esin S., Batoni G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol. 2017;8:2409. doi: 10.3389/fmicb.2017.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebeaux D., Ghigo J.-M., Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78(3):510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theuretzbacher U., Gottwalt S., Beyer P., Butler M., Czaplewski L., Lienhardt C., et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect Dis. 2019;19(2):e40–e50. doi: 10.1016/S1473-3099(18)30513-9. [DOI] [PubMed] [Google Scholar]
- 9.Kang D., Kirienko N.V. Interdependence between iron acquisition and biofilm formation in Pseudomonas aeruginosa. J Microbiol. 2018;56(7):449–457. doi: 10.1007/s12275-018-8114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Post S.J., Shapiro J.A., Wuest W.M. Connecting iron acquisition and biofilm formation in the ESKAPE pathogens as a strategy for combatting antibiotic resistance. Med Chem Commun. 2019;10(4):505–512. doi: 10.1039/C9MD00032A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassat J., Skaar E. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdan A.R., Miyazawa M., Hashimoto K., Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 2016;41(3):274–286. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caza M., Kronstad J. Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front Cell Infect Microbiol. 2013;3:80. doi: 10.3389/fcimb.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson B.R., Bogdan A.R., Miyazawa M., Hashimoto K., Tsuji Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med. 2016;22(12):1077–1090. doi: 10.1016/j.molmed.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muckenthaler M.U., Rivella S., Hentze M.W., Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344–361. doi: 10.1016/j.cell.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellermann M., Arthur J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic Biol Med. 2017;105:68–78. doi: 10.1016/j.freeradbiomed.2016.10.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer J., Özkaya Ö., Kümmerli R. Bacterial siderophores in community and host interactions. Nat Rev Microbiol. 2020;18(3):152–163. doi: 10.1038/s41579-019-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira C.M.H., Vilas-Boas A., Sousa C.A., Soares H.M.V.M., Soares E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Expr. 2019;9:1–12. doi: 10.1186/s13568-019-0796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed E., Holmström S.J.M. Siderophores in environmental research: roles and applications. Microb Biotechnol. 2014;7(3):196–208. doi: 10.1111/1751-7915.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balado M., Lages M.A., Fuentes-Monteverde J.C., Martínez-Matamoros D., Rodríguez J., Jiménez C., et al. The siderophore piscibactin is a relevant virulence factor for Vibrio anguillarum favored at low temperatures. Front Microbiol. 2018;9:1–16. doi: 10.3389/fmicb.2018.01766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llamas M.A., Sparrius M., Kloet R., Jiménez C.R., Vandenbroucke-Grauls C., Bitter W. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J Bacteriol. 2006;188(5):1882–1891. doi: 10.1128/JB.188.5.1882-1891.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brickman T.J., Armstrong S.K. Alcaligin siderophore production by Bordetella bronchiseptica strain RB50 Is not repressed by the BvgAS virulence control system. J Bacteriol. 2002;184(24):7055–7057. doi: 10.1128/JB.184.24.7055-7057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilis A., Khan M.A., Verrijdt W., Taghavi S., Diels L., Mergeay M., et al. In: Global Environmental Biotechnology. Wise D.L., editor. Springer; Dordrecht: 1997. Siderophore alcaligin E production by Alcaligenes eutrophus CH34: features of the ale-operon and application perspectives of alcaligin E for leaching of heavy metals; pp. 61–73. [DOI] [Google Scholar]
- 24.Payne S.M., Niesel D.W., Peixotto S.S., Lawlor K.M. Expression of hydroxamate and phenolate siderophores by Shigella flexneri. J Bacteriol. 1983;155(3):949–955. doi: 10.1128/jb.155.3.949-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley F.C., Marolda C.L., Cheung J., Buac S., Heinrichs D.E., Payne S.M. Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by staphyloferrin A, staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect Immunity. 2011;79(6):2345–2355. doi: 10.1128/IAI.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar V.S., Menon S., Agarwal H., Gopalakrishnan D. Characterization and optimization of bacterium isolated from soil samples for the production of siderophores. Res-Effic Technol. 2017;3(4):434–439. doi: 10.1016/j.reffit.2017.04.004. [DOI] [Google Scholar]
- 27.Cox C.D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa, Infect. Immunity. 1985;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miethke M., Marahiel M.A. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71(3):413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haag H., Hantke K., Drechsel H., Stojiljkovic I., Jung G., Zahner H. Purification of yersiniabactin: a siderophore and possible virulence factor of Yersinia enterocolitica. J Gen Microbiol. 1993;139(9):2159–2165. doi: 10.1099/00221287-139-9-2159. [DOI] [PubMed] [Google Scholar]
- 30.Krewulak K.D., Vogel H.J. Structural biology of bacterial iron uptake. Biochim Biophys Acta. 2008;1778(9):1781–1804. doi: 10.1016/j.bbamem.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Brown J.S., Holden D.W. Iron acquisition by Gram-positive bacterial pathogens. Microbes Infect. 2002;4(11):1149–1156. doi: 10.1016/S1286-4579(02)01640-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith K.F., Oram D.M. In: Encyclopedia of microbiology. Schaechter M., editor. Academic Press; San Diego, CA, USA: 2009. Corynebacteria (including diphtheria) pp. 94–106. [DOI] [Google Scholar]
- 33.Modarresi F., Azizi O., Shakibaie M.R., Motamedifar M., Mosadegh E., Mansouri S. Iron limitation enhances acyl homoserine lactone (AHL) production and biofilm formation in clinical isolates of Acinetobacter baumannii. Virulence. 2015;6(2):152–161. doi: 10.1080/21505594.2014.1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheldon J.R., Skaar E.P. Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog. 2020;16:1–32. doi: 10.1371/journal.ppat.1008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simner P.J., Patel R., Burnham C.-A. Cefiderocol antimicrobial susceptibility testing considerations: the Achilles' heel of the trojan horse? J Clin Microbiol. 2020;59(1) doi: 10.1128/JCM.00951-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syed Y.Y. Cefiderocol: a review in serious gram-negative bacterial infections. Drugs. 2021;81(13):1559–1571. doi: 10.1007/s40265-021-01580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cappellini M.D., Bejaoui M., Agaoglu L., Porter J., Coates T., Jeng M., et al. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta-thalassemia Clin. Ther. 2007;29:909–917. doi: 10.1016/j.clinthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Vichinsky E., Torres M., Minniti C.P., Barrette S., Habr D., Zhang Y., et al. Efficacy and safety of deferasirox compared with deferoxamine in sickle cell disease: two-year results including pharmacokinetics and concomitant hydroxyurea. Am J Hematol. 2013;88(12):1068–1073. doi: 10.1002/ajh.v88.1210.1002/ajh.23569. [DOI] [PubMed] [Google Scholar]
- 39.Yang L.P.H., Keam S.J., Keating G.M. Deferasirox. Drugs. 2007;67:2211–2230. doi: 10.2165/00003495-200767150-00007. [DOI] [PubMed] [Google Scholar]
- 40.Corcé V., Gouin S.G., Renaud S., Gaboriau F., Deniaud D. Recent advances in cancer treatment by iron chelators. Bioorg Med Chem Lett. 2016;26(2):251–256. doi: 10.1016/j.bmcl.2015.11.094. [DOI] [PubMed] [Google Scholar]
- 41.Vessieres A. In: Metal-based anticancer agents. Casini A., Vessières A., Meier-Menches S.M., editors. The Royal Society of Chemistry; 2019. Iron compounds as anticancer agents; pp. 62–90. [DOI] [Google Scholar]
- 42.Richardson D.R. Iron chelators as therapeutic agents for the treatment of cancer. Crit Rev Oncol Hematol. 2002;42(3):267–281. doi: 10.1016/S1040-8428(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 43.Yu Y., Gutierrez E., Kovacevic Z., Saletta F., Obeidy P., Rahmanto Y.S., et al. Iron chelators for the treatment of cancer. Curr Med Chem. 2012;19:2689–2702. doi: 10.2174/092986712800609706. [DOI] [PubMed] [Google Scholar]
- 44.Karp J.E., Giles F.J., Gojo I., Morris L., Greer J., Johnson B., et al. A phase I study of the novel ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) in combination with the nucleoside analog fludarabine for patients with refractory acute leukemias and aggressive myelopro. Leuk Res. 2008;32:71–77. doi: 10.1016/j.leukres.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunos C.A., Waggoner S., von Gruenigen V., Eldermire E., Pink J., Dowlati A., et al. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(4):1298–1306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukushima T., Kawabata H., Nakamura T., Iwao H., Nakajima A., Miki M., et al. Iron chelation therapy with deferasirox induced complete remission in a patient with chemotherapy-resistant acute monocytic leukemia. Anticancer Res. 2011;31:1741–1744. [PubMed] [Google Scholar]
- 47.Miller M.J., Walz A.J., Zhu H., Wu C., Moraski G., Möllmann U., et al. Design, synthesis, and study of a mycobactin−artemisinin conjugate that has selective and potent activity against tuberculosis and malaria. J Am Chem Soc. 2011;133(7):2076–2079. doi: 10.1021/ja109665t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha P., Yeoh B.S., Xiao X., Golonka R.M., Kumarasamy S., Vijay-Kumar M. Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation. Biochem Pharmacol. 2019;168:71–81. doi: 10.1016/j.bcp.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mielich-Süss B., Lopez D. Molecular mechanisms involved in Bacillus subtilis biofilm formation. Environ Microbiol. 2015;17(3):555–565. doi: 10.1111/1462-2920.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landini P., Antoniani D., Burgess J.G., Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl Microbiol Biotechnol. 2010;86(3):813–823. doi: 10.1007/s00253-010-2468-8. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y.-H., Zhang L.-H. Quorum sensing and quorum-quenching enzymes. J Microbiol. 2005;43:101–109. [PubMed] [Google Scholar]
- 52.Singh P.K., Parsek M.R., Greenberg E.P., Welsh M.J. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 53.Lin M.-H., Shu J.-C., Huang H.-Y., Cheng Y.-C. Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS ONE. 2012;7:1–7. doi: 10.1371/journal.pone.0034388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Léséleuc L., Harris G., KuoLee R., Chen W. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(10):5397–5400. doi: 10.1128/AAC.00778-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banin E., Vasil M.L., Greenberg E.P. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci. 2005;102:11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornelis P., Dingemans J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front Cell Infect Microbiol. 2013;3:75. doi: 10.3389/fcimb.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen T., Dong G., Zhang S., Zhang X., Zhao Y., Cao J., et al. Effects of iron on the growth, biofilm formation and virulence of Klebsiella pneumoniae causing liver abscess. BMC Microbiol. 2020;20(1) doi: 10.1186/s12866-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y., Outten F.W. IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J Bacteriol. 2009;191(4):1248–1257. doi: 10.1128/JB.01086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pagès J.-M., James C.E., Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6(12):893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 60.Möllmann U., Heinisch L., Bauernfeind A., Köhler T., Ankel-Fuchs D. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals. 2009;22(4):615–624. doi: 10.1007/s10534-009-9219-2. [DOI] [PubMed] [Google Scholar]
- 61.Tomaras A.P., Crandon J.L., McPherson C.J., Banevicius M.A., Finegan S.M., Irvine R.L., et al. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57(9):4197–4207. doi: 10.1128/AAC.00629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim A., Kutschke A., Ehmann D.E., Patey S.A., Crandon J.L., Gorseth E., et al. Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa: assessing the risk for resistance and attenuated efficacy. Antimicrob Agents Chemother. 2015;59(12):7743–7752. doi: 10.1128/AAC.00831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo T.A., Page M.G.P., Beanan J.M., Olson R., Hujer A.M., Hujer K.M., et al. In vivo and in vitro activity of the siderophore monosulfactam BAL30072 against Acinetobacter baumannii. J Antimicrob Chemother. 2011;66(4):867–873. doi: 10.1093/jac/dkr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paech F., Messner S., Spickermann J., Wind M., Schmitt-Hoffmann A.-H., Witschi A.T., et al. Mechanisms of hepatotoxicity associated with the monocyclic β-lactam antibiotic BAL30072. Arch Toxicol. 2017;91(11):3647–3662. doi: 10.1007/s00204-017-1994-x. [DOI] [PubMed] [Google Scholar]
- 65.Ghazi I., Monogue M., Tsuji M., Nicolau D. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology. 2018;101(5-6):278–284. doi: 10.1159/000487441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonomo R.A. Cefiderocol: a novel siderophore cephalosporin defeating carbapenem-resistant pathogens. Clin Infect Dis. 2019;69:S519–S520. doi: 10.1093/cid/ciz823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monogue M.L., Tsuji M., Yamano Y., Echols R., Nicolau D.P. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother. 2017;61(11) doi: 10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumoto S., Singley C.M., Hoover J., Nakamura R., Echols R., Rittenhouse S., et al. Efficacy of cefiderocol against carbapenem-resistant Gram-negative Bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother. 2017;61(9) doi: 10.1128/AAC.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghazi I.M., Monogue M.L., Tsuji M., Nicolau D.P. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents. 2018;51(2):206–212. doi: 10.1016/j.ijantimicag.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto S., Kanazawa S., Nakamura R., Tsuji M., Sato T., Yamano Y. In vivo efficacy of cefiderocol against carbapenem-resistant Gram-negative Bacilli in murine urinary tract infection models. Open Forum Infect. Dis. 2017;4 doi: 10.1093/ofid/ofx163.1208. S472–S472. [DOI] [Google Scholar]
- 71.Nakamura R., Ito-Horiyama T., Takemura M., Toba S., Matsumoto S., Ikehara T., et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models Antimicrob. Agents Chemother. 2019;63(9) doi: 10.1128/AAC.02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito-Horiyama T., Ishii Y., Ito A., Sato T., Nakamura R., Fukuhara N., et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother. 2016;60(7):4384–4386. doi: 10.1128/AAC.03098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kohira N., West J., Ito A., Ito-Horiyama T., Nakamura R., Sato T., et al. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother. 2016;60(2):729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito A., Nishikawa T., Matsumoto S., Yoshizawa H., Sato T., Nakamura R., et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(12):7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhanel G.G., Golden A.R., Zelenitsky S., Wiebe K., Lawrence C.K., Adam H.J., et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative Bacilli. Drugs. 2019;79(3):271–289. doi: 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 76.Portsmouth S., van Veenhuyzen D., Echols R., Machida M., Ferreira J.C.A., Ariyasu M., et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319–1328. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 77.Wunderink R.G., Matsunaga Y., Ariyasu M., Clevenbergh P., Echols R., Kaye K.S., et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021;21(2):213–225. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 78.Higgs D.R., Engel J.D., Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 79.Rees D.C., Williams T.N., Gladwin M.T. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 80.Urbinati F., Madigan C., Malik P. Pathophysiology and therapy for haemoglobinopathies. Part II: thalassaemias. Expert Rev Mol Med. 2006;8(10):1–26. doi: 10.1017/S1462399406010805. [DOI] [PubMed] [Google Scholar]
- 81.Ribeil J.-A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376(9):848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 82.Poggiali E., Cassinerio E., Zanaboni L., Cappellini M.D. An update on iron chelation therapy. Blood Transfus. 2012;10:411–422. doi: 10.2450/2012.0008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kohgo Y., Ikuta K., Ohtake T., Torimoto Y., Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88(1):7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brittenham G.M. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364(2):146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ballas S.K., Zeidan A.M., Duong V.H., DeVeaux M., Heeney M.M. The effect of iron chelation therapy on overall survival in sickle cell disease and β-thalassemia: a systematic review. Am J Hematol. 2018;93(7):943–952. doi: 10.1002/ajh.v93.710.1002/ajh.25103. [DOI] [PubMed] [Google Scholar]
- 86.Ceci A., Baiardi P., Felisi M., Cappellini M.D., Carnelli V., de Sanctis V., et al. The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br J Haematol. 2002;118:330–336. doi: 10.1046/j.1365-2141.2002.03554.x. [DOI] [PubMed] [Google Scholar]
- 87.Davis B.A., O’Sullivan C., Jarritt P.H., Porter J.B. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 88.Cappellini M.D., Cohen A., Piga A., Bejaoui M., Perrotta S., Agaoglu L., et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 89.Mobarra N., Shanaki M., Ehteram H., Nasiri N., Sahmani M., Saeidi M., et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Stem Cell Res. 2016;10:239–247. https://pubmed.ncbi.nlm.nih.gov/27928480 [PMC free article] [PubMed] [Google Scholar]
- 90.Nisbet-Brown E., Olivieri N.F., Giardina P.J., Grady R.W., Neufeld E.J., Séchaud R., et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361(9369):1597–1602. doi: 10.1016/S0140-6736(03)13309-0. [DOI] [PubMed] [Google Scholar]
- 91.Neufeld E.J. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006;107:3436–3441. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cappellini M.D. Long-term efficacy and safety of deferasirox. Blood Rev. 2008;22:S35–S41. doi: 10.1016/S0268-960X(08)70007-9. [DOI] [PubMed] [Google Scholar]
- 93.Lal A., Porter J., Sweeters N., Ng V., Evans P., Neumayr L., et al. Combined chelation therapy with deferasirox and deferoxamine in thalassemia. Blood Cells Mol Dis. 2013;50(2):99–104. doi: 10.1016/j.bcmd.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cassinerio E., Orofino N., Roghi A., Duca L., Poggiali E., Fraquelli M., et al. Combination of deferasirox and deferoxamine in clinical practice: an alternative scheme of chelation in thalassemia major patients. Blood Cells Mol Dis. 2014;53(3):164–167. doi: 10.1016/j.bcmd.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Tanner M.A., Galanello R., Dessi C., Smith G.C., Westwood M.A., Agus A., et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115(14):1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 96.Lai M.E., Grady R.W., Vacquer S., Pepe A., Carta M.P., Bina P., et al. Increased survival and reversion of iron-induced cardiac disease in patients with thalassemia major receiving intensive combined chelation therapy as compared to desferoxamine alone. Blood Cells Mol Dis. 2010;45(2):136–139. doi: 10.1016/j.bcmd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Totadri S., Bansal D., Bhatia P., Attri S.V., Trehan A., Marwaha R.K. The deferiprone and deferasirox combination is efficacious in iron overloaded patients with β-thalassemia major: a prospective, single center, open-label study, Pediatr. Blood. Cancer. 2015;62(9):1592–1596. doi: 10.1002/pbc.25533. [DOI] [PubMed] [Google Scholar]
- 98.Karami H., Kosaryan M., Hadian Amree A., Darvishi-Khezri H., Mousavi M. Combination iron chelation therapy with deferiprone and deferasirox in iron-overloaded patients with transfusion-dependent β-thalassemia major. Clin Pract. 2017;7(1):11–14. doi: 10.4081/cp.2017.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borgna-Pignatti C., Rugolotto S., De Stefano P., Zhao H., Cappellini M.D., Del Vecchio G.C., et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 100.Hoffbrand A.V., Cohen A., Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102:17–24. doi: 10.1182/blood-2002-06-1867. [DOI] [PubMed] [Google Scholar]
- 101.Rheault M.N., Bechtel H., Neglia J.P., Kashtan C.E. Reversible Fanconi syndrome in a pediatric patient on deferasirox, Pediatr. Blood Cancer. 2011;56(4):674–676. doi: 10.1002/pbc.22711. [DOI] [PubMed] [Google Scholar]
- 102.Rienhoff H.Y., Viprakasit V., Tay L., Harmatz P., Vichinsky E., Chirnomas D., et al. A phase 1 dose-escalation study: safety, tolerability, and pharmacokinetics of FBS0701, a novel oral iron chelator for the treatment of transfusional iron overload. Haematologica. 2011;96(4):521–525. doi: 10.3324/haematol.2010.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neufeld E.J., Galanello R., Viprakasit V., Aydinok Y., Piga A., Harmatz P., et al. A phase 2 study of the safety, tolerability, and pharmacodynamics of FBS0701, a novel oral iron chelator, in transfusional iron overload. Blood. 2012;119:3263–3268. doi: 10.1182/blood-2011-10-386268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parkinson C.J., Birrell G.W., Chavchich M., Mackenzie D., Haynes R.K., de Kock C., Richardson D.R., Edstein M.D. Development of pyridyl thiosemicarbazones as highly potent agents for the treatment of malaria after oral administration. J Antimicrob Chemother. 2019;74:2965–2973. doi: 10.1093/jac/dkz290. [DOI] [PubMed] [Google Scholar]
- 105.Ferrer P., Tripathi A.K., Clark M.A., Hand C.C., Rienhoff H.Y.J., Sullivan D.J.J. Antimalarial iron chelator, FBS0701, shows asexual and gametocyte Plasmodium falciparum activity and single oral dose cure in a murine malaria model. PloSOne. 2012;7 doi: 10.1371/journal.pone.0037171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steegmann-Olmedillas J.L. The role of iron in tumour cell proliferation. Clin Transl Oncol. 2011;13(2):71–76. doi: 10.1007/s12094-011-0621-1. [DOI] [PubMed] [Google Scholar]
- 107.Jung M., Mertens C., Tomat E., Brüne B. Iron as a central player and promising target in cancer progression. Int J Mol Sci. 2019;20(2):273. doi: 10.3390/ijms20020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A., et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(2018):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 109.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu Y.u., Wong J., Lovejoy D.B., Kalinowski D.S., Richardson D.R. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12(23):6876–6883. doi: 10.1158/1078-0432.CCR-06-1954. [DOI] [PubMed] [Google Scholar]
- 111.Kalinowski D.S., Richardson D.R. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev. 2005;57(4):547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 112.Shao J., Zhou B., Di Bilio A.J., Zhu L., Wang T., Qi C., et al. A Ferrous-Triapine complex mediates formation of reactive oxygen species that inactivate human ribonucleotide reductase. Mol Cancer Ther. 2006;5(3):586–592. doi: 10.1158/1535-7163.MCT-05-0384. [DOI] [PubMed] [Google Scholar]
- 113.Kolesar J.M., Schelman W.R., Geiger P.G., Holen K.D., Traynor A.M., Alberti D.B., et al. Electron paramagnetic resonance study of peripheral blood mononuclear cells from patients with refractory solid tumors treated with Triapine. J Inorg Biochem. 2008;102(4):693–698. doi: 10.1016/j.jinorgbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsimberidou A.-M., Alvarado Y., Giles F.J. Evolving role of ribonucleoside reductase inhibitors in hematologic malignancies. Expert Rev Anticancer Ther. 2002;2(4):437–448. doi: 10.1586/14737140.2.4.437. [DOI] [PubMed] [Google Scholar]
- 115.Murren J., Modiano M., Clairmont C., Lambert P., Savaraj N., Doyle T., et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2003;9:4092–4100. [PubMed] [Google Scholar]
- 116.Gojo I., Tidwell M.L., Greer J., Takebe N., Seiter K., Pochron M.F., et al. Phase I and pharmacokinetic study of Triapine, a potent ribonucleotide reductase inhibitor, in adults with advanced hematologic malignancies. Leuk Res. 2007;31(9):1165–1173. doi: 10.1016/j.leukres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 117.Knox J.J., Hotte S.J., Kollmannsberger C., Winquist E., Fisher B., Eisenhauer E.A. Phase II study of Triapine in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161) Invest New Drugs. 2007;25(5):471–477. doi: 10.1007/s10637-007-9044-9. [DOI] [PubMed] [Google Scholar]
- 118.Zeidner J.F., Karp J.E., Blackford A.L., Smith B.D., Gojo I., Gore S.D., et al. A phase II trial of sequential ribonucleotide reductase inhibition in aggressive myeloproliferative neoplasms. Haematologica. 2014;99(4):672–678. doi: 10.3324/haematol.2013.097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kunos C.A., Andrews S.J., Moore K.N., Chon H.S., Ivy S.P. Randomized phase II trial of Triapine-cisplatin-radiotherapy for locally advanced stage uterine cervix or vaginal cancers. Front Oncol. 2019;9:1067. doi: 10.3389/fonc.2019.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma B., Goh B.C., Tan E.H., Lam K.C., Soo R., Leong S.S., et al. A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2008;26(2):169–173. doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 121.Schelman W.R., Morgan-Meadows S., Marnocha R., Lee F., Eickhoff J., Huang W., et al. A phase I study of Triapine in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;63(6):1147–1156. doi: 10.1007/s00280-008-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Traynor A.M., Lee J.-W., Bayer G.K., Tate J.M., Thomas S.P., Mazurczak M., et al. A phase II trial of triapine (NSC# 663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer: Eastern Cooperative Oncology Group Study 1503. Invest New Drugs. 2010;28(1):91–97. doi: 10.1007/s10637-009-9230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuan J., Lovejoy D.B., Richardson D.R. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 124.Lee J.-C., Chiang K.-C., Feng T.-H., Chen Y.-J., Chuang S.-T., Tsui K.-H., et al. The iron chelator, Dp44mT, effectively inhibits human oral squamous cell carcinoma cell growth in vitro and in vivo. Int J Mol Sci. 2016;17(9):1435. doi: 10.3390/ijms17091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nam S.-Y., Han N.-R., Yoon K.W., Kim H.-M., Jeong H.-J. Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT), an anticancer agent, exerts an anti-inflammatory effect in activated human mast cells. Inflamm Res. 2017;66(10):871–879. doi: 10.1007/s00011-017-1067-x. [DOI] [PubMed] [Google Scholar]
- 126.Kovacevic Z., Chikhani S., Lovejoy D.B., Richardson D.R. Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: a new strategy for the treatment of pancreatic cancer. Mol Pharmacol. 2011;80(4):598–609. doi: 10.1124/mol.111.073627. [DOI] [PubMed] [Google Scholar]
- 127.Jansson P.J., Kalinowski D.S., Lane D.J.R., Kovacevic Z., Seebacher N.A., Fouani L., et al. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol Res. 2015;100:255–260. doi: 10.1016/j.phrs.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 128.Lovejoy D.B., Sharp D.M., Seebacher N., Obeidy P., Prichard T., Stefani C., et al. Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J Med Chem. 2012;55(16):7230–7244. doi: 10.1021/jm300768u. [DOI] [PubMed] [Google Scholar]
- 129.Quach P., Gutierrez E., Basha M.T., Kalinowski D.S., Sharpe P.C., Lovejoy D.B., et al. Methemoglobin formation by triapine, di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), and other anticancer thiosemicarbazones: identification of novel thiosemicarbazones and therapeutics that prevent this effect. Mol Pharmacol. 2012;82(1):105–114. doi: 10.1124/mol.112.078964. [DOI] [PubMed] [Google Scholar]
- 130.Guo Z.L., Richardson D.R., Kalinowski D.S., Kovacevic Z., Tan-Un K.C., Chan G.C. The novel thiosemicarbazone, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), inhibits neuroblastoma growth in vitro and in vivo via multiple mechanisms. J Hematol Oncol. 2016;9:98. doi: 10.1186/s13045-016-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salim K.Y., Vareki S.M., Danter W., Koropatnick J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget. 2016;7:41363–41379. doi: 10.18632/oncotarget.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vareki S.M., Salim K.Y., Danter W.R., Koropatnick J. Novel anti-cancer drug COTI-2 synergizes with therapeutic agents and does not induce resistance or exhibit cross-resistance in human cancer cell lines. PloSOne. 2018;13 doi: 10.1371/journal.pone.0191766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hider R.C., Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.