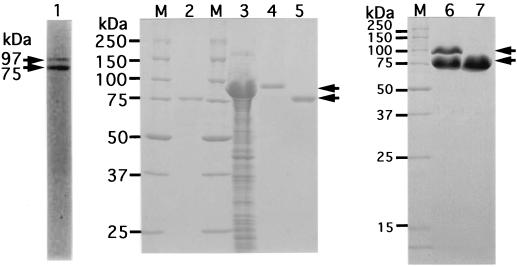
FIG. 1.
Electrophoretic profile of xanthan lyases. The preparations were subjected to SDS-PAGE. Lane M, synthetic polypeptides with molecular masses of 250, 150, 100, 75, 50, 37, 25, and 15 kDa; lane 1, partially purified xanthan lyase (after Sephacryl S-200HR column chromatography) from Bacillus sp. strain GL1; lane 2, purified xanthan lyase (after QAE-Sephadex A-25 column chromatography) from Bacillus sp. strain GL1; lane 3, cell extract of E. coli transformed with xanthan lyase gene; lane 4, purified xanthan lyase (97 kDa) from E. coli; lanes 5 and 7, processed xanthan lyase (75 kDa) from E. coli; lane 6, conversion of 97-kDa enzyme from E. coli to 75-kDa enzyme. Arrows indicate the positions of xanthan lyases.