Abstract
Despite advances, cardiac resynchronisation therapy (CRT) remains fundamentally orientated to the dyssynchrony of left bundle branch block (LBBB), in which septo-lateral electrical and mechanical delays predominate. For non-LBBB patients response rates to conventional CRT are lower and mortality and rehospitalisation rates are not reduced. Despite this, alternative approaches which tailor CRT to the differing dyssynchrony patterns of non-LBBB have yet to be developed. In the specific non-LBBB subgroup of right bundle branch block (RBBB) with left posterior fascicular block (LPFB), ventricular conduction via the left anterior fascicle results in a unique early lateral, and late septal depolarisation, or lateral to septal left ventricular (LV) delay, an electrical sequence which is followed mechanically. This latero-septal delay is somewhat the reverse of LBBB and was overcome by fusing right ventricular (RV) septal pacing with intrinsic conduction via the left anterior fascicle, achieving successful resynchronisation without implantation of a left ventricular lead. A stable fusion pattern was achieved via the ‘Negative AV Hysteresis with Search’ algorithm (Abbott, St Paul, Minnesota). Improvement in all standard CRT response indices was achieved at 3 months: QRS duration was reduced from 153 to 106 ms, ejection fraction increased from 14 to 32%, and LV end-systolic and end-diastolic diameters reduced by 19% and 12.5% respectively. NYHA class improved from III-IV to class II. Cardiac resynchronisation for RBBB with LPFB can be successfully achieved with a standard pacemaker or defibrillator without left ventricular lead implantation by fusing RV septal-only pacing with intrinsic conduction.
1. Introduction
The poorer, and sometimes negative, responses of non-LBBB patients to CRT reported in trials and meta analyses [1] has led some to question “is RBBB [right bundle branch block] an inappropriate indication for CRT or is CRT applied in the wrong way in patients with RBBB?” [2] This question may equally be asked of all non-LBBB subgroups other than just RBBB. In LBBB the mechanical dyssynchrony is one of septo-lateral contraction delay, dictated by the septo-lateral electrical delay of LBBB conduction [1]. Such dyssynchrony may be overcome by CRT, which achieves resynchronisation by fusing multiple pacing sites together (biventricular pacing/multi-site pacing) or LV-only pacing fused with intrinsic septal conduction [3,4]. However, there is no reason to expect this same septo-lateral delay when conduction patterns other than LBBB are present [1]. Applying LBBB-orientated CRT approaches to the heterogeneous non-LBBB subgroups may indeed be the ‘wrong way’, as such pacing strategies are not matched to the various dyssynchrony patterns of non-LBBB subgroups. If CRT is to be confidently offered beyond LBBB then alternative methods which match pacing sites and/or sequences to dyssynchrony patterns appear necessary. As individual subgroup numbers are smaller, case studies such as this are needed to inform possible approaches, which may be specific for each separate subgroup.
2. Dyssynchrony in RBBB with LPFB
The electrical and mechanical dyssynchrony of RBBB with LPFB is distinctly different to LBBB. Ventricular conduction occurs solely via the left anterior fascicle, with initial brisk depolarisation from the lateral LV wall followed in turn by slower LV terminal conduction, delayed septal, and then right ventricular conduction, an electrical sequence which is followed mechanically. Thus, instead of the septo-lateral electrical delay of LBBB the delay in RBBB/LPFB is latero-septal. The resulting mechanical dyssynchrony of RBBB/LPFB together appears not to have not been fully characterised previously, though one echocardiographic study of healthy subjects with isolated LPFB showed a distinct dyssynchrony pattern of delayed posterior papillary muscle contraction with functional mitral regurgitation (FMR) in 13 of 18 patients [5]. FMR was not present in the patient described herein because of prior mitral valve replacement, but LV dyssynchrony in the form of a distinct latero-septal contraction delay was identified via M-mode echocardiography.
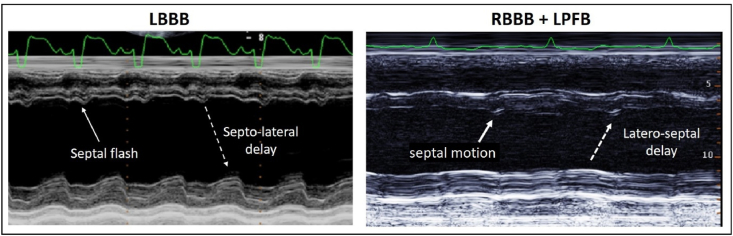
M-mode is an excellent function for visualising contractile sequences [6] and therefore dyssynchrony. In LBBB, M-mode reveals a characteristic ‘septal flash’ or early septal motion, preceding delayed posterolateral wall motion [7]. A reversal of this sequence was revealed in the patient here with RBBB/LPFB. Contraction of the lateral wall preceded septal wall motion by an intraventricular delay of similar magnitude to that seen in LBBB. With this delay septal contraction coincides with the onset of lateral wall relaxation. (Fig. 1).
Fig. 1.
M-mode echocardiogram in LBBB and RBBB/LPFB. In LBBB (left panel) the ‘septal flash’ of early inward septal motion is seen prior to posterolateral wall motion (septo-lateral delay). In RBBB/LPFB (right panel) there is evident hypokinesis, with a contractile sequence that is the reverse of LBBB. Posterolateral wall contraction occurs first, prior to septal motion, and relaxation is underway when septal contraction occurs (latero-septal delay/dyssynchrony). (Left panel adapted from Di Salvo et al. International Journal of Cardiovascular Research [7]. Used with permission).
In keeping with this observed dyssynchrony pattern, synchronising RV-septal pacing to conduction from the lateral insertion site of the left anterior fascicle appeared plausible as a method for restoring synchrony of septal and lateral contraction.
Reported here for the first time is successful cardiac resynchronisation tailored to the unique contractile dyssynchrony of RBBB/LPFB, without requirement for left ventricular lead implantation. Fusing RV septal-only pacing with intrinsic anterior fascicle conduction is analogous to (but the reverse of) the established practice of fusing lateral LV-only pacing with intrinsic septal conduction in LBBB. A stable, optimizable, fusion pattern was achieved by use of the Negative AV Hysteresis with Search algorithm (Abbott, St Paul, Minnesota).
3. Case report
A 71 yo female was admitted in NYHA Class III-IV heart failure. Mitral valve replacement (MVR) for rheumatic valve disease had been performed 10 months earlier, after which the ejection fraction (EF) was 42%. The ECG showed sinus rhythm with first degree AV block (PR 200–220 ms), RBBB and LPFB, with QRS duration 153 ms (Fig. 2). This ECG pattern predated earlier MVR and had been stable for at least 20 months. Neither Electophysiologic Study or Holter Monitoring were performed but postoperative telemetry over 96 hours revealed only minor variations in PR interval and no complete heart block. The patient denied chest pain, cardiac enzymes were normal, and coronary angiography had been normal at the time of MVR. There had been no history of events consistent with myocarditis or viral illness in the interim, and inflammatory markers were not elevated. Transthoracic echocardiogram showed severe dilated cardiomyopathy, global systolic dysfunction, with EF 14%, and increased LV size: Left Ventricular End Diastolic Diameter (LVEDD) 6.6 cm and Left Ventricular End Systolic Diameter (LVESD) 5.8 cm. The mitral valve prosthesis was functioning normally. No abnormalities of the right ventricle or native valves were present. The patient was referred for consideration for device implantation.
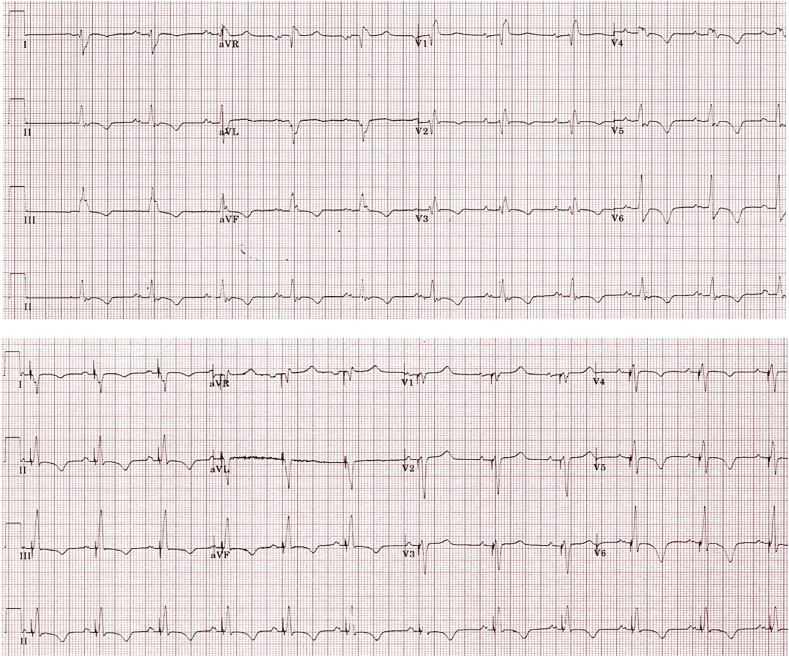
Fig. 2.
Pre- and post-implant ECGs. Top panel: sinus rhythm, first degree block, PR 210 ms. RBBB with LPFB. QRS duration 153 ms. Right axis deviation. Lower panel: RV septal pacing fused with intrinsic conduction via the left anterior fascicle. QRS duration 110 ms here. Note right axis deviation due to the combined conduction via the anterior fascicle (right, inferior axis) plus RVOT pacing (inferior axis).
4. Implant
In the absence of a Class I indication for CRT, a dual chamber (Ellipse™ DR, Abbott) ICD system was implanted. The RV lead was positioned in the right ventricular outflow tract in case of future progression to complete heart block (CHB) and need for chronic pacing. An atrial lead was positioned in the right atrial appendage. Thus, lead positions were chosen on the basis of standard implant considerations rather than any interest in performing resynchronisation.
5. Methods
The Negative AV Hysteresis with Search (NAVHS) algorithm allows RV-septal pacing to be delivered in a desired relationship to intrinsic conduction (before, simultaneously with, or within the QRS). Through this algorithm a desired fusion pattern is kept constant by continual adaptation of the AV pacing delay to changes in intrinsic AV conduction. Algorithm programming options permit relatively simple optimisation of the pattern of fusion.
To function as intended NAVHS requires that the AV delay is programmed longer than the expected AV conduction time (e.g. to 300 ms). This long AV delay functions as a ‘search’ for intrinsic conduction, with searches repeated every 256 beats. If conduction occurs during the search the conduction time is measured and the AV delay is then shortened automatically by a programmable delta (0–120 ms, 10 ms increments). The AV delay also readapts if spontaneous AV conduction emerges outside of the search periods so that the desired fusion pattern is maintained in real time despite variations in conduction. The functional AV delay is therefore not programmed directly but is automated based on current AV conduction time and the Negative AV Hysteresis delta.
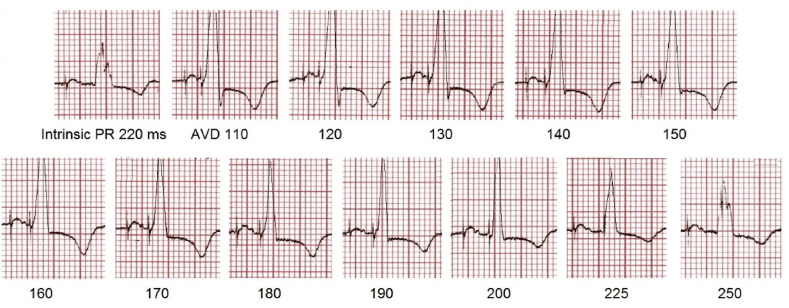
With QRS narrowing as the objective, the narrowest fusion-QRS was observed when RV septal pacing was timed 23 ms prior to the onset of intrinsic conduction (septal preexcitation). Achieving this required 2 simple measurements. First the AV delay at which the narrowest fusion-QRS occurred was determined. During stable AV conduction pacing was commenced at AV delay 110 ms (pure RV pacing) and the AV delay increased in 10 ms steps. Progressive fusion with QRS narrowing occurred, reaching a narrowest QRS at AV delay of 190–200 ms (the fusion-optimised AV delay). (Fig. 3).
Fig. 3.
Determining the AV delay for maximal QRS narrowing. Intrinsic PR interval 220 ms. Pacing is commenced with AV delay 110 ms (pure RV pacing) and increased in 10 ms steps until fusion, with QRS narrowing occurs. The narrowest (optimum) QRS is seen at AV delay 190–200 ms, and again widens with further AV delay increments. (Lead III excerpts from full 12 lead ECG).
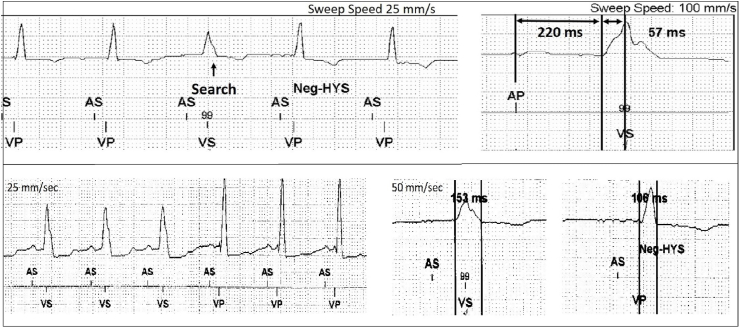
The intrinsic conduction time was then measured from device electrograms with ECG display. Note ‘conduction time’ as used here is the time from P wave onset to the moment of sensing at the right ventricular lead and is therefore comprised of the PR interval plus the time from QRS onset to conduction reaching the RV lead (the QRV). Operationally the NAVHS algorithm measures this interval (277 ms here) and subtracts an AV delta to result in the desired AV delay of 190–200 ms (Fig. 4).
Fig. 4.
NAVHS operation. In the upper panel a single beat AV conduction search (AV delay extension to 300 ms) is applied and intrinsic conduction time measured (upper right): (PR interval 220 ms + QRV interval 57 ms = 277 ms). In the lower panel Negative AV hysteresis (Neg-HYS) is commenced after the first 3 beats of intrinsic rhythm. The AV delay is then shortened by the 80 ms delta, from 277 to 197 ms, resulting in fusion and QRS narrowing from 153 to 106 ms).
Calculation of the NAVHS delta is therefore as follows:
A negative AV delta of 80 ms was programmed resulting in RV septal preexcitation by 23 ms and reduction of QRS duration by 47 ms. Changing the AV delta alters the timing relationship of pacing to intrinsic conduction and is therefore the functional equivalent of the RV-LV timing programming used to optimise the QRS in conventional CRT
6. Results
The QRS duration was reduced from 153 to 106 ms (Fig. 4, lower panel). Minor variations in fusion were evident when the rhythm was atrial paced compared to sinus rhythm, minimised by programming the paced AV delay 25 ms longer than the sensed AV delay. QRS durations across 96 hours of telemetry ranged from 101 to 110 ms. At 3 week review the device AV delay histogram revealed greater than 99% pacing at AV delays of 190–210 ms, This confirms consistent adaptation of AV delays to conduction in both atrial paced and sinus rhythm conduction, and also absence of any periods of CHB.
Initial tolerance of resynchronisation was assessed with walking, with improved distances walked and markedly less dyspnoea with pacing/resynchronisation on compared with pacing off. Twenty-four hours of resynchronisation off versus resynchronisation on was then assessed (blinded). Smartphone step counter recorded 2200 steps in 24 hours with resynchronisation off, with speech-limiting dyspnoea on all walking. Then with resynchronisation on, 4100 steps were recorded in 24 hours, with no speech-limiting dyspnoea, and symptomatic improvement. The patient was discharged with resynchronisation on, pending echocardiogram at 3 weeks.
At 3 weeks the fusion pattern remained unchanged, with >99% fusion pacing. The patient reported improved symptoms, was active, walking a minimum of 4500 steps per day and was in NYHA Class II (from Class III-IV). Transthoracic echocardiogram revealed improvement in ejection fraction from 14 to 25%, and reduction in both LVEDD (12.5%) and LVESV (19%), consistent with CRT-responder categorization. Further improvement in EF to 32% was seen on gated blood pool scan at 3 months.
7. Discussion
Success rates for CRT as it is currently undertaken are lower (or negative) in non-LBBB than in LBBB recipients but alternative approaches which tailor pacing strategies to non-LBBB dyssynchrony patterns have not been reported to date. This case demonstrates a dyssynchrony pattern in RBBB with LPFB which is markedly different to, and largely the reverse of, LBBB. The novel approach of restoring latero-septal synchrony by fusing RV septal-only pacing with left anterior fascicle conduction was successful, achieving all standard measures of CRT response: QRS narrowing, improved ejection fraction and NYHA class, and LV reverse remodelling. Increased activity and reduced heart failure symptoms also occurred. This method requires only a standard pacemaker or ICD implantation and averts the procedural and chronic considerations of left ventricular lead implantation.
The approach of fusing RV septal pacing with intrinsic left anterior fascicle conduction appears logical based on the unique latero-septal delay of RBBB/LPFB and is largely the reverse of the established CRT practice of fusing LV-only pacing with intrinsic septal conduction in LBBB. The Negative AV Hysteresis with Search algorithm appears not to have been used for this purpose previously and proved effective in maintaining the desired fusion pattern over the period studied despite the natural variance in intrinsic AV conduction. Importantly, the programmability of the negative AV delta allows the fusion pattern to be optimised by simple methods to achieve maximal reduction in QRS duration. This method provides an electrical option for RBBB/LPFB patients with heart failure who do not ordinarily qualify for routine CRT implantation. As well, it can be applied for those with existing pacemakers or ICDs who subsequently develop HF, and previous CRT recipients with poor or negative response, as the NAVHS algorithm features on all current and older Abbott pacemakers, ICDs and CRT devices.
Whilst RBBB/LPFB is uncommon, it is more prevalent in the population requiring cardiac devices, and existing CRT methods achieve poorer or negative results. The method used here requires continued function of the left anterior fascicle and in this case RBBB/LPFB had persisted for at least 20 months prior to implant and was still present at follow up.
The success seen in this case compared to historical approaches to biventricular pacing in non-LBBB may be due both to matching of the resynchronisation strategy to the observed dyssynchrony, as well as to retention of the contribution of intrinsic conduction via the anterior fascicle. Conduction from LV pacing sites is often markedly slow, and in replacing the initially brisk anterior fascicle conduction with the slower LV-paced conduction of CRT the degree of QRS narrowing achieved here may not have been possible. Introducing slow LV-paced conduction may also be a factor in the poorer responses in other non-LBBB groups.
In line with conventional CRT, the narrowest QRS was used as the criterion for optimum fusion and selection of the programmed NAVHS delta. It may be that particular ECG morphological endpoints, rather than just QRS narrowing provide different results but this has not been determined.
It may be that the optimum pacing site for RV pacing is elsewhere on the septum or the RV apex. These were not investigated. Instead the RVOT site was selected on standard clinical grounds (risk of progression of CHB with need for chronic ventricular pacing) rather than the interest in investigating resynchronisation. If CHB were to develop subsequently, conduction searches would fail and the device would revert to the programmed long AV delay of 300 ms. The patient would then come under consideration for CRT upgrade. Other changes in AV conduction due to drug treatment or intrinsic AV variations are handled effectively by the algorithm to maintain resynchronisation.
It is difficult to judge whether the newer conduction system pacing approaches via the His Bundle (HB) or the Left Bundle Branch (LBB) would have been effective in this case. For resynchronisation, both are effective at correcting LBBB when pacing sites are distal to the level of block but not when conduction block is more distal to the pacing site [8]. Thus, correction of LPFB may not be achievable if block is distal to HB or LBB pacing sites. Existing reports do not allow assessment of success in correcting LPFB because of trial subgrouping. Fascicular blocks are typically included in the heterogeneous subgroup “Intraventricular Conduction Defects (IVCD)” without specific outcomes for the various IVCDs included. However, compared to LBBB, IVCD subgroups have lower success rates, with less QRS duration reduction and less haemodynamic improvement reported [8]. Failure to correct LPFB may be a contributor to these results. An additional consideration is that when an ICD is to be implanted a three-lead system is still necessary, as a dedicated His or Left Bundle Branch pacing lead is required along with the atrial pacing, and ventricular ICD leads. This adds complexity and hardware compared to the simple placement of a right ventricular septal ICD lead reported here.
No opinion is offered as to the applicability of this approach to other non-LBBB subgroups, including isolated LPFB, in which early septal activation via the right bundle branch would still be present. However, it is hoped that this case study stimulates further investigation into novel approaches to providing CRT in non-LBBB subgroups.
Declaration of competing interest
Malcolm Dennis and Giada Capitani are employees of Abbott Medical.
Dr Paul Sparks has no disclosures to declare.
Financial disclosure
There are no financial disclosures to make. No funding applies.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Cunnington C., Kwok C.S., Duwarkan K., et al. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology. Heart. 2015;101:1456–1462. doi: 10.1136/heartjnl-2014-306811. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio A., Lumens J., Prinzen F.W. Does cardiac resynchronization therapy benefit patients with right bundle branch block. Cardiac resynchronization therapy has a role in patients with right bundle branch block. Circ Arrhythm Electrophysiol. June 2014:532–541. doi: 10.1161/CIRCEP.113.000628. [DOI] [PubMed] [Google Scholar]
- 3.Breithardt G. MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy): cardiac resynchronization therapy towards early management of heart failure. Eur Heart J. 2009;30(21):2551–2553. doi: 10.1093/eurheartj/ehp383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibault B., Ducharme A., Harel F., White M., O'Meara E., Guiertin M.C., Lavoie J., Frasure-Smith N., Dubuc M., Guerra P., et al. Left ventricular versus simultaneous biventricular pacing in patients with heart failure and a QRS complex > 120 milliseconds. Circulation. 2011;124:2874–2881. doi: 10.1161/CIRCULATIONAHA.111.032904. [DOI] [PubMed] [Google Scholar]
- 5.Lopes V.M., Lopes M.G., De Paduai F. Left posterior hemiblock. A new cause of mitral valve prolapse. Adv Cardiol. 1977;19:120–126. doi: 10.1159/000399637. Karger, Basel, [DOI] [PubMed] [Google Scholar]
- 6.Sakamaki F., Seo Y., Atsumi A., Yamamoto M., Machino-Ohtsuka T., Kawamura R., Yamasaki H., Igarashi M., Sekiguchi Y., Ishizu T., Aonuma K. Novel dyssynchrony evaluation by M-mode imaging in left bundle branch block and the application to predict responses for cardiac resynchronization therapy. J Cardiol. 2014;64:199–206. doi: 10.1016/j.jjcc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Di Salvo G., Manea W., Bulbui Z., Issa Z., Fadel B., Soufi B., Ahmadi M., Fayyadh M. Echocardiography in selecting pediatric patients and congenital heart disease patients for resynchronisation therapy. Int J Cardiovasc Res. 2014;3(5) doi: 10.4172/2324-8602.1000182[. [DOI] [Google Scholar]
- 8.Sharma P.S., Vijayaraman P. Conduction system pacing for cardiac resynchronisation. Arrhythm Electophysiol Rev. 2021 Apr;10(1):51–58. doi: 10.15420/aer.2020.45. doi: 10.15420/aer.2020.45. [DOI] [PMC free article] [PubMed] [Google Scholar]