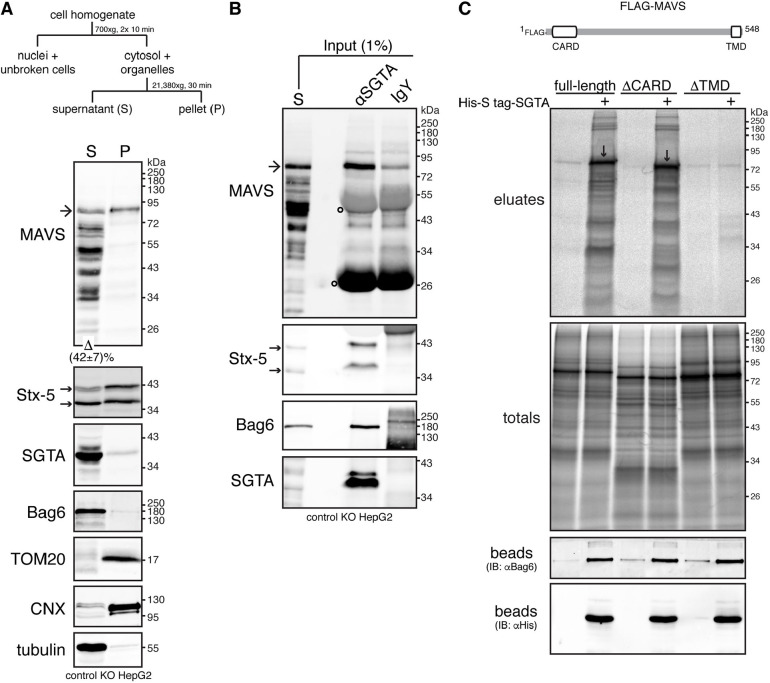
Fig. 2.
SGTA interacts with MAVS. (A) A cytosolic pool of endogenous MAVS can be observed at steady-state. Top, schematic of subcellular fractionation protocol used to separate the cell homogenate into crude cytosolic supernatant (S) and membrane-associated pellet (P) fractions. Bottom, detergent-free extracts from control KO cells (see Fig. S1) were fractionated as shown above. Equivalent amounts of each fraction were analysed by immunoblotting for MAVS and various compartmental markers. Bag6, SGTA and tubulin (cytosolic markers), TOM20 (mitochondrial outer membrane marker) and calnexin (CNX, ER membrane marker) serve as fractionation controls. Note that the MAVS-specific antibody, raised against amino acids 1–135 of human MAVS, detected the ∼80 kDa full-length MAVS (marked by an arrow) and multiple shorter variants that most likely represent C-terminally degraded products or processed forms of the full-length protein (see also Seth et al., 2005). Quantification of the levels of full-length MAVS recovered in the cytosolic fraction is indicated below the MAVS blot. Value represents mean±s.e.m. from three independent experiments. (B) MAVS co-immunoprecipitates with SGTA. The supernatant (S) fraction from A was subjected to immunoprecipitations with equal amounts of chicken anti-SGTA antibody (αSGTA) or chicken IgY antibody (control for non-specific binding). Input and immunoprecipitates were analysed by immunoblotting for the indicated endogenous proteins. Bag6 served as positive control for SGTA binding. In A and B, arrows next to the Stx-5 blots indicate the two Stx-5 isoforms. Open circles on MAVS blots indicate signals derived from denatured antibody heavy and light chains. Blots representative of three independent experiments. (C) In vitro translated MAVS interacts with recombinant SGTA via its transmembrane domain (TMD). Top, schematic of FLAG–MAVS displaying its N-terminal caspase activation and recruitment domain (CARD) and C-terminal TMD. Bottom, FLAG-MAVS full-length, ΔCARD or ΔTMD truncated variants were translated in vitro in the absence or presence (+) of 2 µM His-S-tag-SGTA. A 10% sample of the total translation products was subjected to denaturing immunoprecipitations with anti-FLAG antibody (totals), while the rest was incubated with HisPur cobalt resin and bound proteins were eluted using imidazole (eluates). Totals and eluates were resolved by SDS-PAGE and results visualised by phosphorimaging. Downward arrows indicate full-length and ΔCARD FLAG–MAVS selectively bound by His-S-tag-SGTA. His-S-tag-SGTA and its binding partners within rabbit reticulocyte lysate were released from the resin by incubating the beads with SDS sample buffer (beads) and samples were analysed by immunoblotting (IB). The anti-His and anti-Bag6 immunoblots indicate uniform binding of Bag6 binding-competent His-S-tag-SGTA to beads. Results shown in C are representative of two independent experiments.