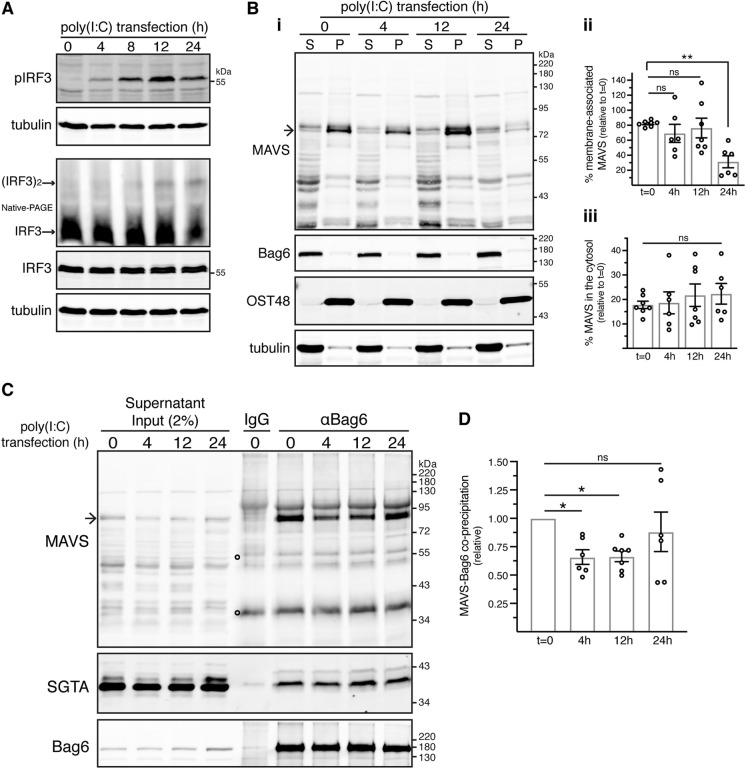
Fig. 6.
Stimulation with poly(I:C) compromises the Bag6–MAVS interaction. (A) Kinetics of IRF3 activation in response to cytosolic poly(I:C). Control KO cells were mock-transfected (t=0) or transfected with poly(I:C) for various times before immunoblotting for the indicated proteins. Activation of endogenous IRF3 was assessed by induction of its phosphorylation and dimerisation. Phosphorylated IRF3 blot is representative of six independent experiments. Western blot for the detection of IRF3 dimer is representative of two independent experiments. pIRF3, phosphorylated IRF3; (IRF3)2, IRF3 dimer. (B) Stimulation with cytosolic poly(I:C) does not grossly alter the levels of MAVS in the crude cytosolic supernatant fraction. (i) Control KO cells were mock-transfected (t=0) or transfected with poly(I:C) for various times before their fractionation as shown in Fig. 2A. The resulting supernatant (S) and pellet (P) fractions were analysed by immunoblotting for the indicated endogenous proteins. (ii,iii) Mean±s.e.m. of the (ii) pellet/total ratio and (iii) supernatant/total ratio of MAVS levels in poly(I:C)-transfected cells relative to the respective ratios in mock-transfected cells (t=0) for six independent experiments as in Bi. **P<0.01; ns, not significant (ordinary one-way ANOVA with Dunnett's multiple comparison tests). (C) Cytosolic poly(I:C) impairs Bag6–MAVS interaction. Supernatant fractions from Bi were subjected to immunoprecipitations with rabbit anti-Bag6 antibody (αBag6) or rabbit control IgG antibody. Inputs and immunoprecipitates were analysed by immunoblotting for the indicated endogenous proteins. SGTA served as loading control as well as internal control for comparable Bag6 binding. Arrow in MAVS blot in Bi and C indicates full-length MAVS. Open circles on MAVS blots indicate signals derived from denatured antibody heavy and light chains. (D) Mean±s.e.m. of MAVS levels that co-immunoprecipitate with Bag6 in poly(I:C)-transfected relative to mock-transfected cells (t=0) for six independent experiments as shown in C. *P<0.05; ns, not significant (one-way ANOVA with Dunnett's multiple comparison tests).