Fig. 6 |. Design strategy for well-structured porphyrin binding proteins.
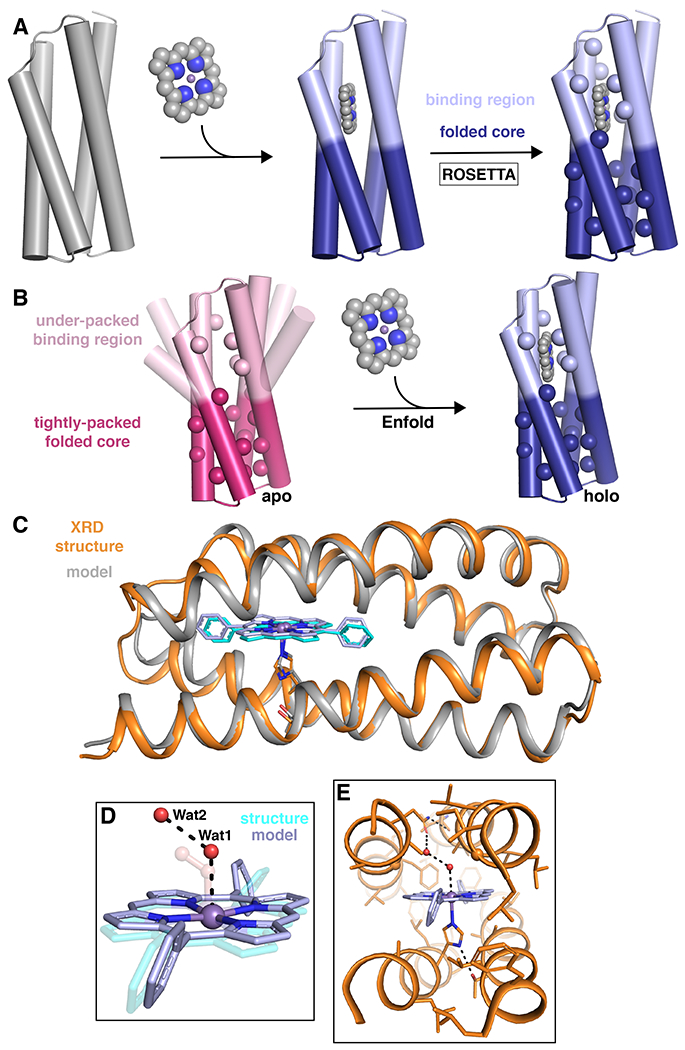
a |The general Enfold design strategy for PS1 in which the binding region and folded core regions are designed simultaneously to give an optimized sequence and backbone around the desired metallocofactor.210 b |An illustration of the Enfold strategy in which the under-packed binding site becomes well-structured on binding the metallocofactor to produce a well-folded, stable holo protein.210 c–e |Structural comparison of the designed model of MPP1 (gray) and the crystal structure (PDB: 7JRQ; orange).213 c |A cartoon representation showing an extremely good backbone match between the design and structure (0.6 Å all backbone RMSD). d |A comparison of the placement of two water molecules (Wat1 and Wat2; red spheres) relative to the dioxygen unit in the design (transparent) and e |extended H-bonding network from the binding site to the surface by the Wat1-Wat2 water network. XRD = X-ray diffraction. Parts a and b adapted from ref.210, Springer Nature Limited. Parts c–e reprinted with permission from ref.213, ACS.
