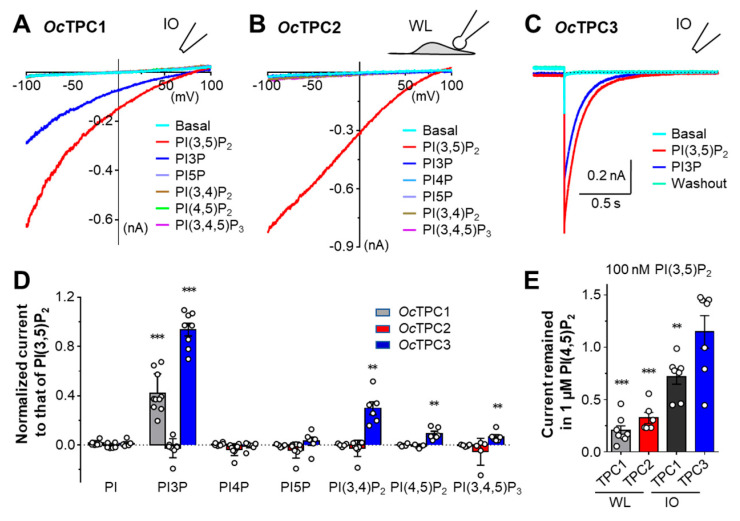
Figure 3.
Rabbit TPC1, TPC2, and TPC3 display different selectivities for phosphoinositides. HEK293 cells transiently transfected with cDNA coding for OcTPC1-EGFP, OcTPC2-EGFP, or OcTPC3-EGFP as indicated were used for voltage-clamp recording using either inside-out (IO, for TPC1 and TPC3) or whole-endolysosome (WL, for TPC2) configurations. (A) Representative I–V curves derived from voltage ramps for OcTPC1 in an IO patch exposed to 100 nM indicated phosphoinositides in the bath. (B) Representative I–V curves derived from voltage ramps for OcTPC2 in a WL patch exposed to 1 µM indicated phosphoinositides in the bath. (C) Representative current traces of OcTPC3 detected in an IO patch exposed to 1 µM indicated phosphoinositides in the bath. The voltage was stepped from the holding potential at 0 mV to +100 mV for 4 s before returning to 0 mV. Only the last 0.3 s of the +100-mV step and the tail at 0 mV are shown for clarity. Dashed line indicates zero current. (D) Summary of peak currents at −100 mV (for TPC1 and TPC2) and tail currents at 0 mV (for TPC3) elicited by the indicated phosphoinositides normalized to that by PI(3,5)P2. Data are means ± SEM for n = 3–11 patches. White circles represent individual data points. ** p < 0.01, *** p < 0.001, different from the theoretical mean of 0 by one-sample t-test. (E) Current in the presence of 100 nM PI(3,5)P2 plus 1 µM PI(4,5)P2 normalized to that in 100 nM PI(3,5)P2 for WL and IO patches obtained from cells that expressed OcTPC1, OcTPC2, or OcTPC3 as indicated. For TPC1 and TPC2, currents at −100 mV obtained from voltage ramps were analyzed; for TPC3, tail currents from 0 mV following the step to +100 mV were analyzed. Data are means ± SEM for n = 6–8 patches. ** p < 0.01, *** p < 0.001, different from the theoretical mean of 1 by one-sample t-test.