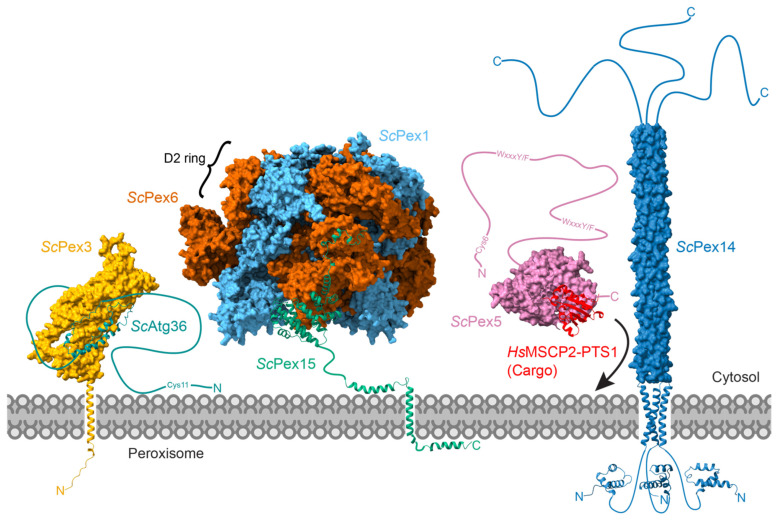
Figure 6.
Putative Pex1/Pex6 substrates (cytoPex15, Pex5, and Atg36) have disordered tails (curved lines) capable of accessing the Pex1/Pex6 D2 pore. Pex1/Pex6 is recruited to the peroxisome membrane by Pex15 (models based on EMDB-6359; PDB 5VXV; Alphafold2 multimer). In vitro, the truncated cytosolic domain of Pex15 is a Pex1/Pex6 substrate. Pex5 bound to a PTS1-tagged protein (model based on HsPEX5 bound to MSCP2 PDB: 2C0L; Alphafold2 ScPex5 AF: P35056) embeds in the DTM, primarily composed of Pex5 bound to Pex14 (modeled from EMDB-12047 [10.2 Å]; Alphafold2 AF: P53122). Pex1/Pex6 is then thought to extract mono-ubiquitinated Pex5 from the membrane. Atg36 interacts with Pex1/Pex6 indirectly through Pex3 and Pex15 (models based on Alphafold2 multimer). Pex1/Pex6 prevents Atg36 phosphorylation, though the mechanism is unclear. Figures made with ChimeraX 1.3 [147].