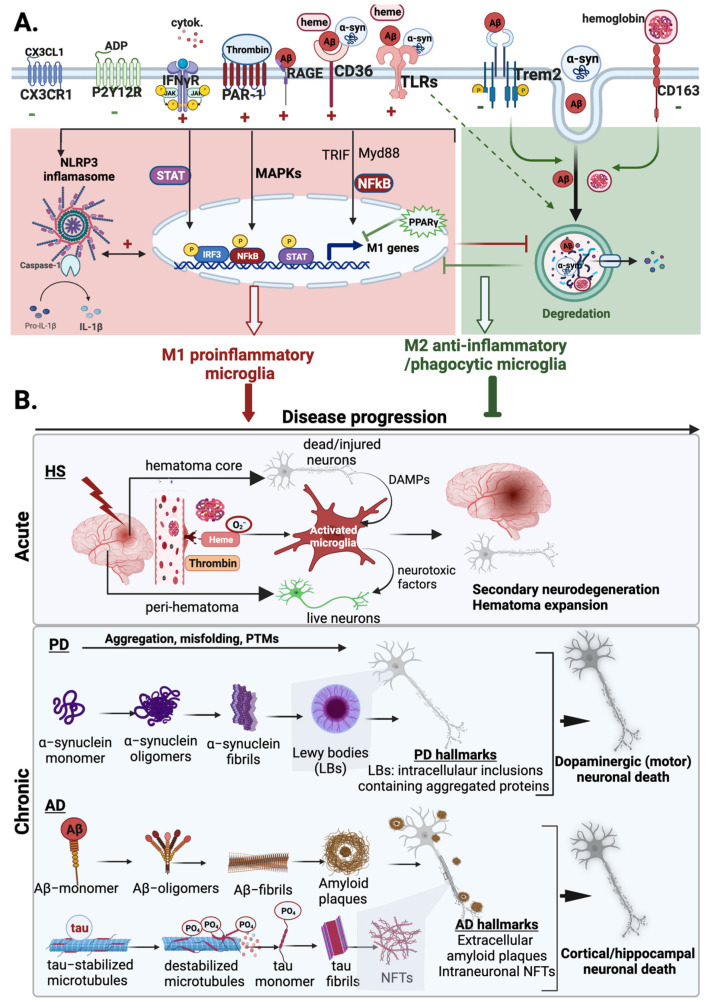
Figure 3.
Microglial functions and diverse activation responses in neurodegenerative diseases. (A) Summary of the major activation mediators and receptors involved in microglial responses to Alzheimer’s disease (AD), Parkinson’s disease (PD), and hemorrhagic stroke (HS). Microglia can be activated directly by disease-specific triggers such as amyloid-β (Aβ) in AD, α-synuclein (α-syn) in PD, or hematoma products such as heme/hemoglobin and thrombin in HS. Various shared microglial receptors interact with these pathogenic molecules, and their overall response depends on the nature of the disease, the stage of the disease, and the specific molecular trigger. Microglial response is also modulated by other mediators such as cytokines present in the cerebrospinal fluid, damage-associated molecules (DAMPs), such as ADP, released from injured neurons, and neuron-derived endogenous signals such as CX3CL1. In general, microglial response can be pro-inflammatory/neurotoxic (+) or anti-inflammatory/neuroprotective (−). Activation of PAR-1, RAGE, CD36, and TLR receptors generally promotes pro-inflammatory responses by activating a range of pro-inflammatory pathways, such as JAK/STAT, MAPK, or NFκB, as well as the NLRP3 inflammasome. These diverse pro-inflammatory pathways can also interact synergistically, promoting chronic neuroinflammation. In contrast, activation of P2YR12 and CX3CR1 negatively regulates microglial activation and pro-inflammatory responses and generally mediates protective functions. Similarly, activation of TREM2 and other scavenger receptors induces microglial phagocytosis of Aβ and other pathogenic proteins, aiding in their clearance. These two generally distinct microglial responses also antagonize one another; degradation of abnormal proteins and pathogenic mediators helps limit chronic neuroinflammation, while pro-inflammatory responses can compromise the microglial phagocytosis response. (B) Summary of the role of microglia phenotypes in the progression of neurodegenerative diseases. In HS, microglia can be activated by thrombin or heme or indirectly by DAMPs from injured neurons, and a sustained microglial pro-inflammatory response contributes to secondary neurodegeneration in areas outside the initial injury zone. Pro-inflammatory microglial responses in PD and AD trigger post-translational modifications (PTMs) and misfolding and aggregation of α-syn and Aβ, that can contribute to the formation of amyloid plaques and Lewy bodies, thereby contributing to disease progression. Pro-inflammatory microglia also induce tau phosphorylation and subsequent aggregation, which promotes neurofibrillary tangle formation (NFT) and overall disease progression. Furthermore, pro-inflammatory microglia exhibit a dysregulated phagocytosis response, limiting the clearance of hematoma products and misfolded proteins that contribute to disease progression. On the contrary, anti-inflammatory/phagocytic microglia phenotypes mediate protective roles and limit disease progression by promoting tissue recovery. Figure created using BioRender.com (accessed on 14 April 2022).