FIGURE 2.
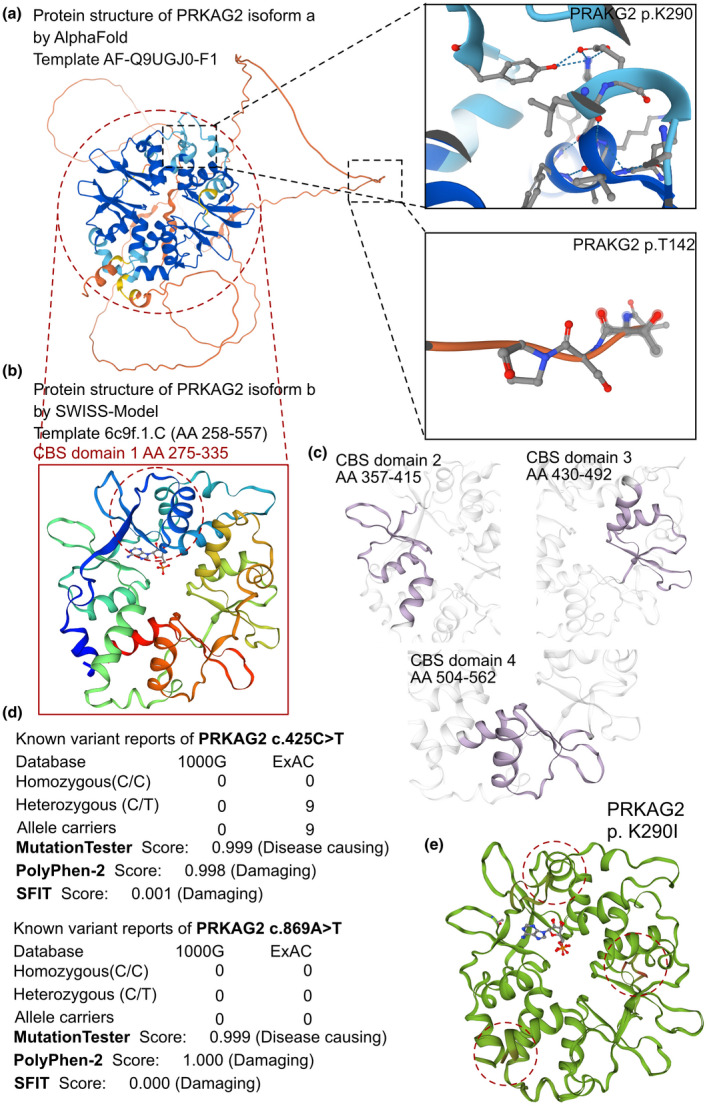
The effects of PRKAG2 c.425C > T and c.869A > T variants on the molecular structure of the protein. (a) the AlphaFold protein structure database was used to predict the PRKAG2 wild‐type protein crystal structure. Four important CBS domains were revealed and were clearly involved in AMPK signal transduction. (b) Protein structure of PRKAG2 isoform b (transcript‐b exon5‐exon16). This region contains four CBS domains with two tandem pairs. (c) Individual crystal structures of CBS domains not involved in the variant reported in this study. (d) The prevalence of PRKAG2 variants of c.425C > T and c.869A > T. (e) SWISS‐MODEL prediction of the wild‐type and p.K290I‐variant protein crystal structures. Changes of structure are indicated in the transporter region
