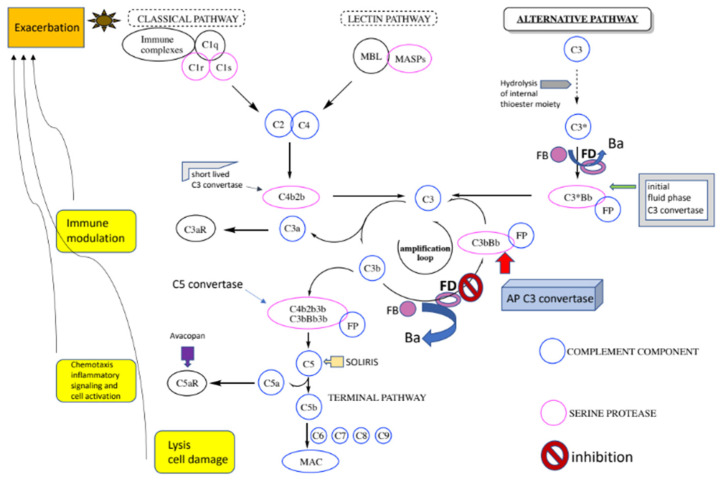
Figure 1.
The complement system with a focus on the AP: A spontaneous hydrolysis of a thioester bond within C3 (C3 “tickover”) results in a conformationally altered C3 (designated C3 (H2O) or C3*) that makes the recruitment of FB possible. FB undergoes its own conformational change and is cleaved by another serine protease, factor D (FD), to generate fragments Ba and Bb. The latter contains the serine protease domain of FB and remains bound to the complex designated C3*Bb and the initial fluid phase AP C3 convertase. This convertase catalyzes the cleavage of C3 molecules to generate fragments C3a (anaphylatoxin) and C3b (opsonin). The same C3 proteolytic cleavage can also be catalyzed by the CP/LP C3 convertases, C4b2b. The released C3b components can enter into the critical amplification loop of the AP where complexes of foreign surface bound C3b and FB are similarly cleaved by FD to generate the predominant AP C3 convertases, C3bBb, on the targeted surfaces capable of additional C3 proteolytic cleavage. The C3b components also have the option of binding to either of the C3 convertases to form the respective C5 convertase complexes responsible for the breakdown of C5 molecules to release C5a and C5b. The latter interacts with C6, C7, C8, and C9 to form the membrane attack complex (MAC), which punctures the surface of some pathogens, resulting in subsequent cell lysis.