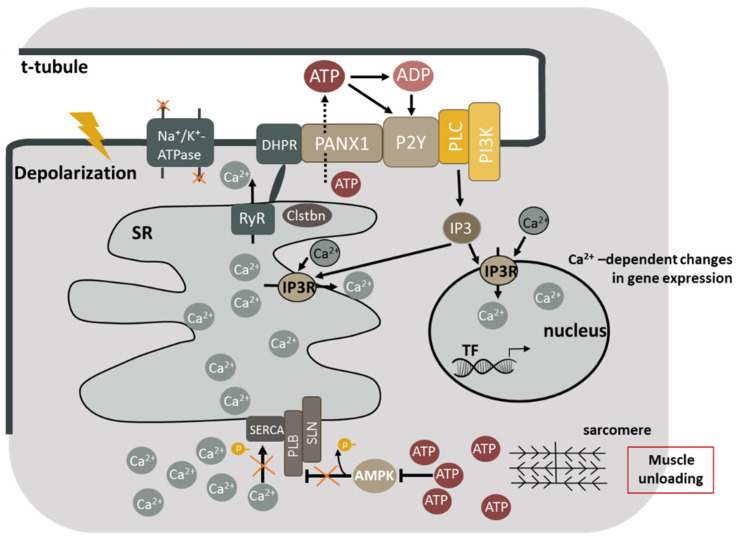
Figure 1.
Molecular mechanisms contributing to the sarcoplasmic calcium accumulation during skeletal muscle unloading. Inactivation of Na,K-ATPase leads to sarcolemma depolarization, which in turn causes the activation of DHPR and the opening of RyR. Sarcoplasmic calcium accumulation leads to the detachment of calstabin from the ryanodine receptor and the occurrence of calcium leakage. Meanwhile, the inactivation of AMP-dependent protein kinase leads to dephosphorylation of PLN, and the blockage of SERCA. Excessive accumulation of calcium ions in the sarcoplasm causes calcium-dependent proteolysis and changes in gene expression in muscle fibers. ATP is released by PANX1 under functional muscle unloading. ATP is rapidly degraded to ADP, AMP, and adenosine under the action of ectonucleotidases. ATP and ADP can affect P2Y receptors associated with G proteins that in turn activate PI3 kinase. PI3 kinase catalyzes phosphorylation of phosphatidylinositol diphosphate (PIP2), giving PIP3 a highly charged residue, which recruits phospholipase C into the membrane, triggering the formation of inositol-1,4,5-triphosphate (IP3). IP3 then binds to IP3 receptors (IP3R) present both in the nuclear envelope and in the sarcoplasmic network, causing a weak signal of calcium release both in the cytosol and in the nucleoplasm, which contributes (probably, with other signaling cascades) to the activation of transcription factors (TF) leading to the expression or repression of the genes involved in the phenotype of muscle cells. DHPR—dihydropyridine channels; RyR—ryanodine receptors; SERCA—sarcoplasmic calcium-dependent ATPase; Clstbn—calstabin; AMPK—AMP-dependent protein kinase; PANX1—pannexin channels; P2Y—P2Y receptors; G—g-protein; PI3K—PI3-kinase; IP3R—IP3 receptors (IP3R—inositol 1,4,5-triphosphate receptors); IP3—inositol 1,4,5-triphosphate; PLC—phospholipase C.