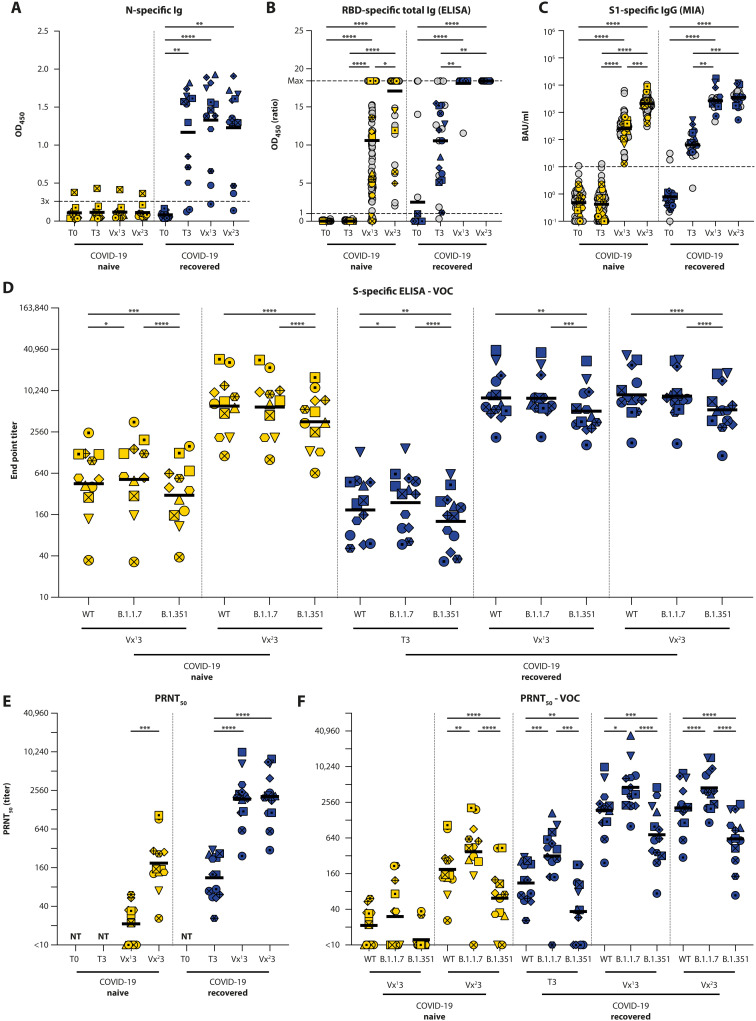
Fig. 2. Detection of SARS-CoV-2–specific humoral responses.
Total Ig levels were measured in COVID-19–naive (yellow) and –recovered (blue) donors at the acute, convalescent, post-vaccination 1, and post-vaccination 2 stage (T0, T3, Vx13, and Vx23) by an (A) ELISA against nucleocapsid (N) and (B) RBD. (C) Quantitative IgG against S1 was measured by a Luminex bead assay. (D) Antibody binding to WT SARS-CoV-2 and VOC B.1.1.7 and B.1.351 was determined by end point titration in ELISA. Virus neutralization was measured by PRNT50 against (E) WT SARS-CoV-2 (D614G) and (F) VOC. Analyses in (B) and (C) were performed on 121 participants, and in-depth analyses were performed in (A), (D), (E), and (F) on 25 participants. Time points in (A), (B), and (C) were compared by performing a nonparametric repeated measures Friedman test. End point titers between VOC in (D) were compared by RM one-way ANOVA or Friedman test. PRNT50 titers in (D) and (E) were compared by RM one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Symbol shapes indicate individual donors and are consistent throughout the figures. Lines in (A) and (B) show the means; lines in (C), (D), (E), and (F) show geometric means. Dotted lines represent cutoff values for positivity [3× background OD450 in (A), OD450 ratio = 1 in (B), and 10.08 BAU/ml in (C)]. NT: not tested.