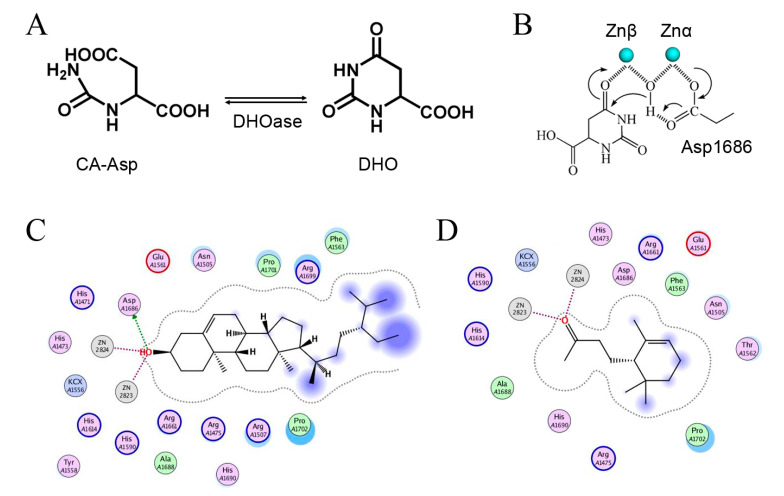
Figure 7.
Inhibition of dihydroorotase by S. purpurea-root-acetone. (A) DHOase catalyzes the reversible cyclization of N-carbamoyl aspartate (CA-asp) to dihydroorotate (DHO), for the biosynthesis of pyrimidine nucleotides. (B) The reaction mechanism of DHOase. This enzyme contains a binuclear metal center (Znα/Znβ) and a residue Asp1686 crucial for catalysis. The hydrolysis of DHO undergoes three steps: the hydrolytic water molecule must be activated for the nucleophilic attack, the amide bond of the substrate must be made more electrophilic by polarization of the carbonyl oxygen bond, and the leaving-group nitrogen must be protonated as the carbon–nitrogen bond is cleaved. (C) Docking of stigmast-5-en-3-ol in the active site of huDHOase via the MOE-Dock tool. The docking model showed that the binding of stigmast-5-en-3-ol involved Znα, Znβ, and Asp1686 within the active site of huDHOase. (D) Docking of 7,8-dihydro-α-ionone in the active site of huDHOase via the MOE-Dock tool. The docking model showed the binding of 7,8-dihydro-α-ionone involved Znα and Znβ within the active site of huDHOase.