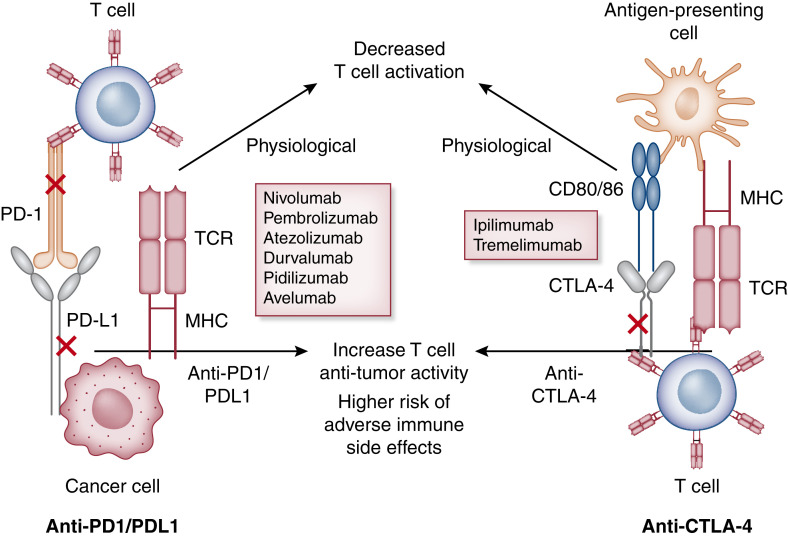
Figure 1.
Mechanism of action of immune checkpoint inhibitors. Cytotoxic T lymphocyte–associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) signaling networks at homeostasis. Integration of both positive and negative costimulatory signals during and after the initial T cell activation will determine the fate and intensity of the alloimmune response. For CTLA-4 (right panel), the first step in antigen recognition is the binding of the antigen to MHC molecules on the antigen-presenting cell and creating a complex with the T cell receptor (TCR) located on the T cell. This is followed by the interaction of the CD28 molecule with B7 (CD 80/86) initiating a costimulatory signal, leading to further T cell stimulation (this is in addition to other costimulatory molecules not depicted here). As a negative feedback process to prevent overstimulation, T cell activation leads to the upregulation of the CTLA-4 molecule, which competes with the B7-CD28 ligand and, in turn, leads to T cell arrest, thus providing brakes to the immune system. CTLA-4 antagonist binds to the CTLA-4 molecule and prevents it from binding to B7, leading to the sustained activation of the T cell (lifting the foot off the brakes). Similarly, binding of the PD-1 (left panel) molecule with programmed cell death ligand 1 (PD-L1) and PD-L2 leads to an inhibitory signal with decreased effector T cell function, suppressing immune surveillance and permitting neoplastic growth. PD-1 inhibitors bind to the PD-1 molecule, preventing its interaction with PD-L1/L2 and thus leading to continued T cell stimulation (pressing on the accelerator). Majority of the data support the role of increased PD-L1 expression in human tumors and serve as the biomarker to consider PD-1 inhibitors for treatment.