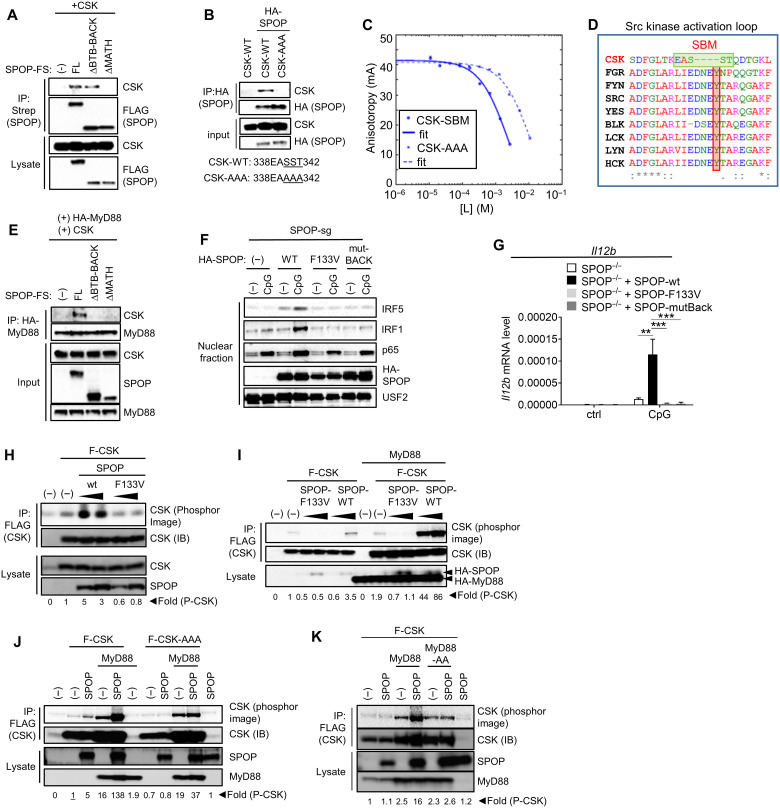
Fig. 7. SPOP acts as adaptor protein to recruit CSK into the TLR signaling complex.
(A and B) IP/IB analysis of HEK293T cells based on exogenously expressed proteins. The SBM in CSK and its mutation in CSK-AAA are indicated. (C) FA competition binding assay. Peptides containing the candidate SBM of CSK and the mutated sequence CSK-AAA compete with fluorescein-Puc91–106 for binding to the SPOP MATH domain. [L] is the peptide concentration. Continuous lines are nonlinear least-squares fits to a complete competitive binding model. See Table 1 for Kd values. (D) Clustal Omega–based sequence alignment of Src family kinases and CSK. The SBM and the conserved tyrosine are highlighted by green and red boxes, respectively. (E) IP/IB analysis of HEK293T cells based on exogenously expressed proteins. (F) Nuclear translocation assay of SPOP-deficient RAW264.7 cells that were reconstituted with indicated forms of SPOP. (G) qPCR analysis of cells described in (F) that were stimulated with CpG-DNA for 4 hours. (H to K) In vitro kinase assays based on proteins expressed in HEK293T cells. CSK-AAA, SST340/341/341AAA. Fold differences of CSK autophosphorylation as determined by phosphor image analysis. The activity used for reference (set as 1) is underlined. Data represent mean ± SD from three independent experiments. **P < 0.01, ***P < 0.005 are determined by two-way ANOVA with Sidak’s multiple comparison test (G).