Abstract
Background
Tixagevimab–cilgavimab is a neutralising monoclonal antibody combination hypothesised to improve outcomes for patients hospitalised with COVID-19. We aimed to compare tixagevimab–cilgavimab versus placebo, in patients receiving remdesivir and other standard care.
Methods
In a randomised, double-blind, phase 3, placebo-controlled trial, adults with symptoms for up to 12 days and hospitalised for COVID-19 at 81 sites in the USA, Europe, Uganda, and Singapore were randomly assigned in a 1:1 ratio to receive intravenous tixagevimab 300 mg–cilgavimab 300 mg or placebo, in addition to remdesivir and other standard care. Patients were excluded if they had acute organ failure including receipt of invasive mechanical ventilation, extracorporeal membrane oxygenation, vasopressor therapy, mechanical circulatory support, or new renal replacement therapy. The study drug was prepared by an unmasked pharmacist; study participants, site study staff, investigators, and clinical providers were masked to study assignment. The primary outcome was time to sustained recovery up to day 90, defined as 14 consecutive days at home after hospital discharge, with co-primary analyses for the full cohort and for participants who were neutralising antibody-negative at baseline. Efficacy and safety analyses were done in the modified intention-to-treat population, defined as participants who received a complete or partial infusion of tixagevimab–cilgavimab or placebo. This study is registered with ClinicalTrials.gov, NCT04501978 and the participant follow-up is ongoing.
Findings
From Feb 10 to Sept 30, 2021, 1455 patients were randomly assigned and 1417 in the primary modified intention-to-treat population were infused with tixagevimab–cilgavimab (n=710) or placebo (n=707). The estimated cumulative incidence of sustained recovery was 89% for tixagevimab–cilgavimab and 86% for placebo group participants at day 90 in the full cohort (recovery rate ratio [RRR] 1·08 [95% CI 0·97–1·20]; p=0·21). Results were similar in the seronegative subgroup (RRR 1·14 [0·97–1·34]; p=0·13). Mortality was lower in the tixagevimab–cilgavimab group (61 [9%]) versus placebo group (86 [12%]; hazard ratio [HR] 0·70 [95% CI 0·50–0·97]; p=0·032). The composite safety outcome occurred in 178 (25%) tixagevimab–cilgavimab and 212 (30%) placebo group participants (HR 0·83 [0·68–1·01]; p=0·059). Serious adverse events occurred in 34 (5%) participants in the tixagevimab–cilgavimab group and 38 (5%) in the placebo group.
Interpretation
Among patients hospitalised with COVID-19 receiving remdesivir and other standard care, tixagevimab–cilgavimab did not improve the primary outcome of time to sustained recovery but was safe and mortality was lower.
Funding
US National Institutes of Health (NIH) and Operation Warp Speed.
Introduction
Neutralising monoclonal antibodies targeting SARS-CoV-2 are effective for treatment of early COVID-19 among outpatients with risk factors for progression to severe illness,1, 2, 3 as well as for primary prevention4 and post-exposure prophylaxis.5, 6 Hospitalised patients might also benefit from neutralising monoclonal antibodies, although previous results have varied by agent and serostatus.7, 8, 9 Monoclonal antibody effectiveness is threatened, however, by the emergence of new SARS-CoV-2 variants.10, 11, 12, 13, 14, 15
Tixagevimab–cilgavimab (AZD7442 [Evusheld], consisting of AZD8895 and AZD1061; AstraZeneca) is a combination of two Fc-modified human monoclonal antibodies derived from B cells from two individuals who had recovered from SARS-CoV-2 infection. These antibodies recognise non-overlapping sites on the receptor binding domain of the SARS-CoV-2 spike glycoprotein.16 The Fc-modifications extend half-life and reduce FcR and C1q complement binding to minimise the theoretical risk of antibody-dependent enhancement of disease.17, 18, 19
The ACTIV-3–TICO (Accelerating COVID-19 Therapeutic Interventions and Vaccines–Therapeutics for Inpatients with COVID-19) platform protocol evaluates promising antiviral therapies among patients hospitalised with COVID-19, for whom outcomes, including mortality, remain poor.20 The first three monoclonal antibody products studied were halted at the early futility analyses on the basis of a seven-category ordinal scale for pulmonary function, as described previously.8, 9, 21 Here we report the results of the ACTIV-3–TICO trial comparing tixagevimab–cilgavimab versus placebo, in patients receiving remdesivir and other standard care.
Research in context.
Evidence before this study
Mortality is common among patients hospitalised with COVID-19, despite improvements in standard care (including remdesivir, dexamethasone, other immune modulators, and anticoagulants). Neutralising monoclonal antibodies selectively bind to SARS-CoV-2 spike protein, suppress viral replication in vitro and in animal models, and prevent clinical progression and hospitalisation when given early to high-risk outpatients with COVID-19. Treatment of hospitalised, seronegative individuals with the monoclonal antibody combination casirivimab–imdevimab significantly reduced mortality in the RECOVERY trial. However, SARS-CoV-2 omicron variants are fully resistant to this combination monoclonal antibody and show reduced susceptibility to others. Tixagevimab–cilgavimab, a combination of two monoclonal antibodies with an approximately 90-day half-life, prevented symptomatic SARS-CoV-2 infection when given as pre-exposure prophylaxis, reduced hospitalisations by more than 50% when given during early COVID-19 in participants who are not hospitalised, and maintained antiviral activity against omicron variants. This is the first phase 3 study evaluating the efficacy of tixagevimab–cilgavimab in patients who are hospitalised.
Added value of this study
In this phase 3 trial, we evaluated a single intravenous dose of tixagevimab–cilgavimab, in addition to remdesivir and other standard care. Tixagevimab–cilgavimab did not improve the primary endpoint of sustained patient recovery, but it was safe and led to a clinically relevant reduction in mortality. The mortality signal was numerically larger in patients requiring high-flow oxygen or non-invasive mechanical ventilation at study entry and in patients infected with the delta SARS-CoV-2 variant. In contrast to some previous monoclonal antibody studies, there were no differences in any of the efficacy or safety study outcomes by baseline endogenous neutralising antibody serostatus.
Implications of all the available evidence
A single intravenous dose of tixagevimab–cilgavimab might provide additional clinical benefits to current standard care in patients hospitalised with COVID-19. Benefits might actually be greatest in patients with more advanced respiratory failure. The mortality reduction observed in this study belong to a secondary endpoint and thus requires additional validation. As baseline serostatus was not associated with efficacy or safety outcomes, antibody testing might not be required to administer this treatment.
Methods
Study design
The TICO platform protocol outlines the testing of multiple candidate antiviral therapies via a phase 3 multi-arm, multistage, double-blind, randomised controlled trial design at 81 sites in the USA, Europe, Uganda, and Singapore (see appendix p 30).20 The protocol was approved by a governing institutional review board for each enrolling site. Written informed consent for trial participation was obtained from each enrolled patient or a legally authorised representative, as applicable.20
Study participants
Adult, hospitalised patients were eligible if they had confirmed SARS-CoV-2 infection and symptoms for up to 12 days. Patients were excluded if they had acute organ failure including receipt of invasive mechanical ventilation, extracorporeal membrane oxygenation, vasopressor therapy, mechanical circulatory support, or new renal replacement therapy. At the beginning of the trial (Feb 10, 2021), patients on high-flow nasal oxygen or non-invasive ventilation were excluded. On July 19, 2021, after 743 (51%) of 1455 patients were enrolled, eligibility was expanded at the recommendation of the US Food and Drug Administration and data and safety monitoring board (DSMB) to include these patients. A full list of eligibility criteria and rationale for change during the trial is provided in the appendix (pp 17, 18).
Randomisation and masking
Participants were enrolled by study investigators or a designee. Hospitalised adults with COVID-19 were randomly assigned in a 1:1 ratio to either tixagevimab 300 mg–cilgavimab 300 mg or placebo. Randomisation was done via an interactive web-based application with a computer-generated random sequence and was stratified by study site pharmacy. Study drug was prepared by an unmasked pharmacist on site; study participants, site study staff, investigators, and clinical providers were masked to trial group assignment. Masking was further assured by placing a coloured sleeve over the infusion bags used for tixagevimab–cilgavimab and placebo.
Procedures
Tixagevimab and cilgavimab were derived from convalescent B cells from two COVID-19 survivors.16 They are both YTE (M257Y, S259T, T261E) modified isoforms to extend their half-life and therapeutic treatment window.17, 18 Additionally, their Fc regions have three amino acid substitutions (L234F, L235E, P331S) that reduce binding to human Fc receptors and C1q to minimise the theoretical risk of Fc- or complement-mediated antibody-dependent enhanced disease.19
Adults hospitalised with COVID-19 were randomly assigned in a 1:1 ratio to either tixagevimab 300 mg–cilgavimab 300 mg, administered as a single intravenous infusion over a 30-min period, or placebo (appendix p 16).
Presence of anti-spike SARS-CoV-2 neutralising plasma antibody at baseline was centrally measured by batch on cryopreserved plasma by means of the GenScript SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript, Piscataway, NJ, USA) with 30% binding inhibition to define positivity per manufacturer specifications. Additional plasma antibody (BioRad Platelia, Hercules, CA, USA; Quanterix Simoa, Billerica, MA, USA) and antigen (Quanterix Simoa) assays are detailed in the appendix (pp 22, 23).
Remdesivir was provided to all study participants unless contraindicated. Receipt of exogenous SARS-CoV-2 antibodies (eg, other monoclonal antibody products, convalescent plasma, or hyperimmune globulin) was prohibited. Corticosteroids were encouraged for participants with hypoxaemia. Other medications were permitted as part of locally determined standard of care. National Institutes of Health (NIH) treatment guidelines were encouraged but not required by the protocol.22
Outcomes
The primary outcome was time from randomisation to sustained clinical recovery up to day 90, defined as return to home for 14 consecutive days (with home defined as the participant's residence type before hospitalisation or a location that provided similar or less intensive medical care). This outcome captures patient-centred, clinically important events after hospital discharge, given that hospital discharge might occur quickly particularly during pandemic conditions and the omission of post-discharge events might underestimate disease burden. Key secondary efficacy outcomes included all-cause mortality up to day 90 and a composite of sustained recovery and mortality up to day 90. Other secondary outcomes are seven-category pulmonary ordinal outcome scale, a seven-category pulmonary-plus ordinal outcome scale, time to discharge from the index hospitalization, a composite safety outcomes through days 5, 28 and 90, reactions associated with the infusion of the study treatments (appendix pp 26–29).
The main safety outcome was a composite of death, serious adverse events, incident organ failure, and serious co-infections up to day 90. A local site investigator graded all adverse events for severity using the toxicity table of the Division of AIDS from the National Institute of Allergy and Infectious Diseases, version 2.1. Adverse events were categorised according to codes in the Medical Dictionary for Regulatory Activities (MedDRA), version 23.1 and grouped by system organ class; definitions, timing, and additional outcomes are described in the appendix (pp 26–29).
Statistical analysis
The study was initially planned to provide 90% power to detect a recovery rate ratio (RRR) of 1·25 (25% improved rate of sustained recovery in the tixagevimab–cilgavimab group compared with placebo) at the 0·05 (two-sided) level of significance. This required 843 primary events (ie, participants who achieved sustained recovery), which was estimated to be achieved with 1000 participants. Motivated by the results of other trials7, 8, 9 suggesting that participants without endogenous SARS-CoV-2 antibodies at baseline (seronegative) might benefit from neutralising monoclonal antibody treatment, whereas seropositive participants might not benefit and might even be harmed, the study was amended on Aug 19, 2021, to provide 90% power to detect an RRR of 1·20 overall and of 1·28 among seronegative participants. On the basis of intermediate pooled data, this was estimated to be achieved with 1228 primary events wherein 56% would be seronegative and contribute 545 events.
The primary analysis tested hypotheses for the primary outcome in the full cohort and the seronegative subgroup simultaneously as co-primary. Holm's method23 was used to control family-wise type 1 error at 0·05 by first testing the hypothesis with the lower p value at the 0·025 (two-sided) level of significance and then if significant, the hypothesis with the higher p value at 0·05. Power estimates were updated on the basis of the final enrolment and analysis plan (appendix p 23). There was no adjustment for multiple comparisons of secondary outcomes.
The primary analysis was modified intention-to-treat, restricted to participants who received a complete or partial infusion of tixagevimab–cilgavimab or placebo. Because treatment assignment was blinded, the reasons for not receiving an infusion were independent from the treatment assignment. For the primary outcome of sustained recovery, participants who were alive but had not had sustained recovery were censored at the last date on which the endpoint status was ascertained. Participants who withdrew consent were censored at the date of withdrawal. We compared treatment groups for time to sustained recovery using a Fine-Gray model (accounting for the competing risk of death), stratified by study site pharmacy and country (7 strata total).24, 25, 26 We compared death and composite safety outcomes up to day 90 using stratified Cox proportional hazards models and a composite of sustained recovery and mortality using the win-ratio method (appendix p 24).27
RRR greater than 1 denotes superiority of tixagevimab–cilgavimab compared with placebo on the cumulative incidence of sustained recovery. Treatment comparisons by means of Cox proportional hazards models are presented such that hazard ratios (HRs) less than 1 (for death and safety outcomes) denote superiority of tixagevimab–cilgavimab. Additional preplanned subgroup and secondary analyses are described in the appendix (pp 26–29). We did all statistical analyses using SAS (version 9.4) and R (version 4.1). The trial was overseen by an independent DSMB who evaluated unmasked interim data for futility, efficacy, and safety. This study is registered with ClinicalTrials.gov, NCT04501978.
Role of the funding source
The Division of Clinical Research at the National Institute of Allergy and Infectious Diseases funded this project. Investigators from NIH were directly involved in all aspects of this study, including study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit.
Results
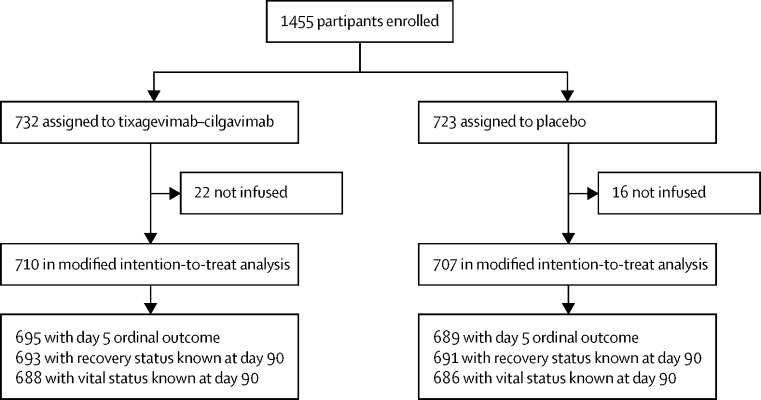
From Feb 10 to Sept 30, 2021, we enrolled 1455 patients at 81 sites in the USA, Europe, Uganda, and Singapore. The primary analysis population included 1417 patients who received a complete or partial infusion of tixagevimab–cilgavimab (n=710) or placebo (n=707; figure 1 , appendix p 30). Baseline characteristics were balanced between groups and the overall study group was generally representative of patients hospitalised with COVID-19 (table 1 , appendix pp 32–37). The median duration of symptoms at enrolment was 8 days (6–10), 1041 (73%) were unvaccinated, and 128 (9%) were immunocompromised including 56 (4%) taking antirejection medications. Common pre-randomisation medications administered included remdesivir (897 [63%]) and corticosteroids (1035 [73%]; table 1, appendix pp 34, 35). Including those who initiated therapy after study infusion, 1315 (93%) participants received remdesivir. Anti-spike neutralising antibodies were not detectable at baseline for 644 (47%) of 1363 participants (ie, they were seronegative), whereas 1287 (94%) of 1363 had detectable plasma SARS-CoV-2 nucleocapsid antigen concentrations. On the basis of PCR data, 687 (51%) of 1347 were infected with the delta SARS-CoV-2 variant, of which 648 (94%) were enrolled July–September, 2021.
Figure 1.
Trial profile
Table 1.
Baseline characteristics of the primary cohort
| Tixagevimab–cilgavimab group (n=710) | Placebo group (n=707) | ||
|---|---|---|---|
| Age, years | 55 (44–66) | 55 (44–66) | |
| Sex | |||
| Female | 299 (42%) | 295 (42%) | |
| Male | 411 (58%) | 412 (58%) | |
| Race or ethnicity | |||
| Non-Hispanic White | 360 (51%) | 344 (49%) | |
| Non-Hispanic Black | 177 (25%) | 175 (25%) | |
| Hispanic | 119 (17%) | 135 (19%) | |
| Asian | 34 (5%) | 24 (3%) | |
| Other | 20 (3%) | 29 (4%) | |
| Body-mass index in kg/m2 | |||
| 30–39·9 | 281 (40%) | 268 (38%) | |
| ≥40·0 | 102 (14%) | 106 (15%) | |
| Co-existing chronic illness* | |||
| Any | 415 (58%) | 445 (63%) | |
| Hypertension treated with medication | 292 (41%) | 300 (42%) | |
| Diabetes treated with medication | 183 (26%) | 187 (26%) | |
| Asthma | 68 (10%) | 70 (10%) | |
| Renal impairment | 63 (9%) | 70 (10%) | |
| Chronic obstructive pulmonary disease | 44 (6%) | 42 (6%) | |
| Immunocompromised† | 57 (8%) | 71 (10%) | |
| SARS-CoV-2 vaccination status‡ | |||
| Fully vaccinated | 103 (15%) | 101 (14%) | |
| Partially vaccinated | 82 (12%) | 90 (13%) | |
| Not vaccinated | 525 (74%) | 516 (73%) | |
| Days since symptom onset | 8 (6–10) | 8 (6–10) | |
| Medication use before randomisation | |||
| Remdesivir | 447 (63%) | 450 (64%) | |
| Corticosteroid | 518 (73%) | 517 (73%) | |
| Immunomodulator§ | 64 (9%) | 50 (7%) | |
| Antirejection medication | 24 (3%) | 32 (5%) | |
| Therapeutic dose anticoagulation¶ | 58 (8%) | 66 (9%) | |
| Prophylactic or intermediate dose anticoagulation | 467 (66%) | 470 (66%) | |
| Pulmonary ordinal scale category‖ | |||
| Not receiving supplemental oxygen | 174 (25%) | 155 (22%) | |
| Conventional supplemental oxygen <4 L/min | 241 (34%) | 270 (38%) | |
| Conventional supplemental oxygen ≥4 L/min | 216 (30%) | 200 (28%) | |
| High flow nasal cannula or non-invasive ventilation** | 79 (11%) | 82 (12%) | |
| Delta variant†† | 344/685 (50%) | 343/662 (52%) | |
| Genscript neutralising anti-spike antibody positive‡‡ | 380/687 (55%) | 339/676 (50%) | |
| BioRad anti-nucleocapsid antibody positive§§ | 417/687 (61%) | 444/677 (66%) | |
| Quanterix anti-spike immunoglobulin G positive¶¶ | 357/681 (52%) | 349/675 (52%) | |
| Nucleocapsid antigen concentration‖‖ | 1622 (299–4891) | 1675 (247–5287) | |
| Positive (concentration ≥3 pg/mL) | 645/687 (94%) | 642/676 (95%) | |
Data are median (IQR) or n (%).
Full list of co-existing chronic illness in the appendix (p 33).
Immunocompromised is defined as receiving anti-rejection medications, biologic medications to treat autoimmune disease or cancer (excluding interleukin[IL]-1, IL-6, janus kinase [JAK] inhibitors, and tumour necrosis factor [TNF] inhibitors), human immunodeficiency virus, or other immunosuppressive condition.
Fully vaccinated is primary vaccine series dose(s) completed at least 14 days before the onset of symptoms; partial vaccinated is primary vaccine series dose(s) completed within 14 days before onset of symptoms, or one dose received of a two-dose series; not vaccinated is first dose of vaccine received after onset of symptoms or no known vaccine doses received (eight with unknown vaccination status: two in the tixagevimab–cilgavimab group, six in the placebo group).
Immunomodulators. Overall, 58 participants received a JAK inhibitor, 41 received a IL-6 inhibitor, one received a IL-1 inhibitor, and one received a TNF inhibitor (see also appendix p 34).
Therapeutic anticoagulation was defined as receipt of therapeutic doses of heparin, warfarin, or a direct acting oral anticoagulant.
For participants on chronic supplemental oxygen therapy before COVID-19, categorisation on the pulmonary ordinal scale was based on oxygen flow rates above the pre-COVID-19 oxygen flow rate.
On July 19, 2021, enrolment expanded to include participants receiving high flow nasal cannula or non-invasive ventilation.
SARS-CoV-2 delta variant was established from a mid-turbinate swab at baseline based on RT-PCR detection of the N-terminal domain of the delta spike. Of participants infected with the delta SARS-CoV-2 variant, 94% were enrolled July–September, 2021.
GenScript cPass surrogate SARS-CoV-2 neutralisation assay (anti-spike); positive was defined as ≥30% binding inhibition.
BioRad Platelia anti-nucleocapsid assay (total antibody); positive was defined as ≥1·0 sample:cutoff ratio.
Quanterix Simoa anti-spike assay (immunoglobulin G); positive was defined as ≥770 ng/mL.
Quanterix Simoa nucleocapsid antigen; positive was defined as ≥3 pg/mL.
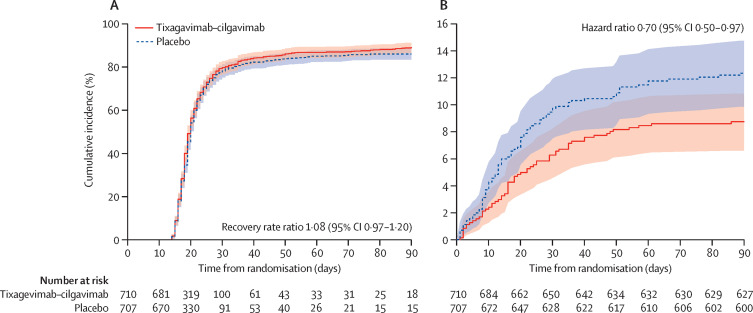
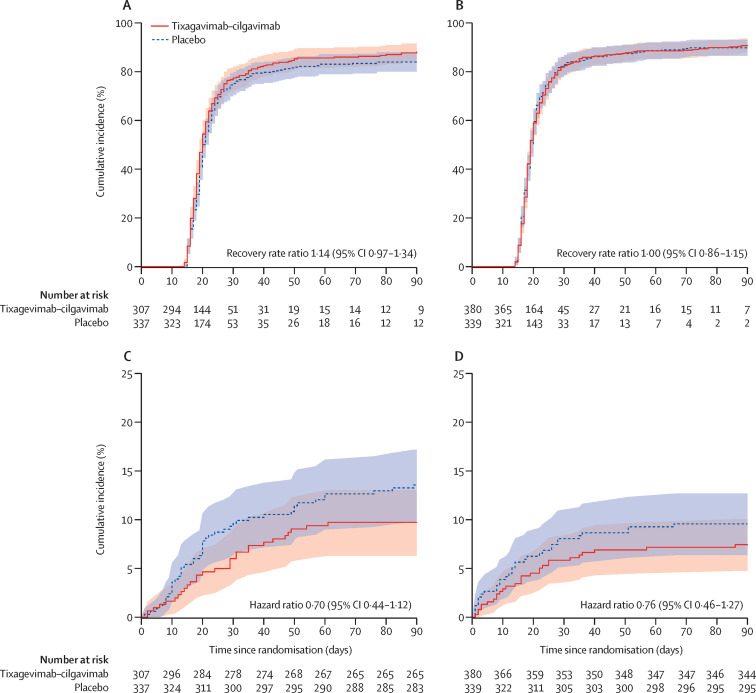
The estimated cumulative incidence of sustained recovery was 78% for the tixagevimab–cilgavimab group and 76% for the placebo group participants at day 28 and 89% and 86%, respectively, at day 90 for the full cohort (RRR 1·08 [95% CI 0·97–1·20]; p=0·21; table 2 , figure 2 ). Results were similar in the seronegative subgroup (RRR 1·14 [0·97–1·34]; p=0·13; table 2; figure 3 ). Results were not materially different in a sensitivity analysis that defined sustained recovery as both being at home and no longer on new supplemental oxygen for 14 consecutive days (data not shown).
Table 2.
Main outcomes for primary cohort and by baseline neutralising antibody status
| Tixagevimab–cilgavimab group (n=710) | Placebo group (n=707) | Rate or hazard ratio*(95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Full cohort | ||||||
| Co-primary, sustained recovery up to day 90†‡ | 617 (89%) | 595 (86%) | 1·08 (0·97–1·20) | 0·21 | ||
| Censored | 34 (5%) | 31 (4%) | .. | .. | ||
| Died before sustained recovery | 59 (8%) | 81 (11%) | .. | .. | ||
| Death up to day 90 | 61 (9%) | 86 (12%) | 0·70 (0·50–0·97) | 0·032 | ||
| Composite safety outcome up to day 90§ | 178 (25%) | 212 (30%) | 0·83 (0·68–1·01) | 0·059 | ||
| Serious adverse event | 34 (5%) | 38 (5%) | .. | .. | ||
| Death or serious adverse event | 85 (12%) | 112 (16%) | .. | .. | ||
| Death, serious adverse event, or organ failure | 166 (23%) | 200 (28%) | .. | .. | ||
| Anti-spike neutralising antibody negative¶ | n=307 | n=337 | .. | .. | ||
| Co-primary, sustained recovery up to day 90† | 260 (85%) | 277 (82%) | 1·14 (0·97–1·34) | 0·13 | ||
| Censored | 20 (7%) | 20 (6%) | .. | .. | ||
| Died before sustained recovery | 27 (9%) | 40 (12%) | .. | .. | ||
| Death up to day 90 | 29 (9%) | 45 (13%) | 0·70 (0·44–1·12) | 0·14 | ||
| Composite safety outcome up to day 90§ | 87 (28%) | 116 (34%) | 0·81 (0·62–1·08) | 0·15 | ||
| Anti-spike neutralising antibody positive¶ | n=380 | n=339 | .. | .. | ||
| Sustained recovery up to day 90 | 340 (90%) | 297 (88%) | 1·00 (0·86–1·15) | 0·95 | ||
| Censored | 12 (3%) | 10 (3%) | .. | .. | ||
| Died before sustained recovery | 28 (7%) | 32 (9%) | .. | .. | ||
| Death up to day 90 | 28 (7%) | 32 (9%) | 0·76 (0·46–1·27) | 0·30 | ||
| Composite safety outcome up to day 90§ | 80 (21%) | 80 (24%) | 0·87 (0·64–1·19) | 0·38 | ||
Recovery rate ratio or hazard ratio, according to the methods and statistical analysis plan in the appendix (p 16). Recovery rate ratios of >1 for sustained recovery and hazard ratios of <1 for death and safety endpoints favour tixagevimab–cilgavimab.
Co-primary outcomes analysed by Holm's method to control familywise type 1 error at 0·05, according to the methods and statistical analysis plan in the appendix (p 16).
Based on the estimated cumulative incidence of sustained recovery, which was 78% for tixagevimab–cilgavimab and 76% for placebo group participants at d ay 28 and 89% and 86%, respectively, at day 90 (figure 2.
Composite of death, serious adverse events, incident organ failure, and serious co-infection.
Neutralising antibody status determined by GenScript cPass surrogate SARS-CoV-2 neutralisation assay (anti-spike); positive was defined as ≥30% binding inhibition. Available for 1363 of 1417 participants in the full cohort.
Figure 2.
Time to sustained recovery (A) and death (B) up to day 90 for the full cohort
RRR and HR calculated according to methods and statistical analysis plan in the appendix (p 20). RRR >1 for sustained recovery and HR <1 for death favour tixagevimab–cilgavimab. By definition, sustained recovery can be achieved no earlier than 14 days after randomisation. The proportional hazards assumption was met; on the basis of an interaction term with log time, 0·209 +/- 0·176 (p=0·24) for sustained recovery and 0·054 +/- 0·161 (p=0·74) for death. HR=hazard ratio. RRR=recovery rate ratio.
Figure 3.
Time to sustained recovery and death up to day 90 by baseline serostatus
(A) Sustained recovery for seronegative cohort. (B) Sustained recovery for seropositive cohort. (C) Death for seronegative cohort. (D) Death for seropositive cohort. RRR and HR calculated according to methods and statistical analysis plan in the appendix (p 23). RRR >1 for sustained recovery and HR <1 for death favour tixagevimab–cilgavimab. By definition, sustained recovery can be achieved no earlier than 14 days after randomisation. HR=hazard ratio. RRR=recovery rate ratio.
Up to day 90, death occurred in 61 (9%) participants in the tixagevimab–cilgavimab group compared with 86 (12%) in the placebo group in the full cohort (HR 0·70 [95% CI 0·50–0·97]; p=0·032; table 2; figure 2); estimates were similar in the seronegative subgroup (29 [9%] vs 45 [13%]; HR 0·70 [0·44–1·12]; p=0·14; table 2; figure 3). In the composite analysis of sustained recovery and death, tixagevimab–cilgavimab outcomes were not significantly different from placebo (win ratio 1·08 [95% CI 0·92–1·27], p=0·33 for overall cohort; 1·16 [0·92–1·47], p=0·21 for the seronegative subgroup; appendix p 40).
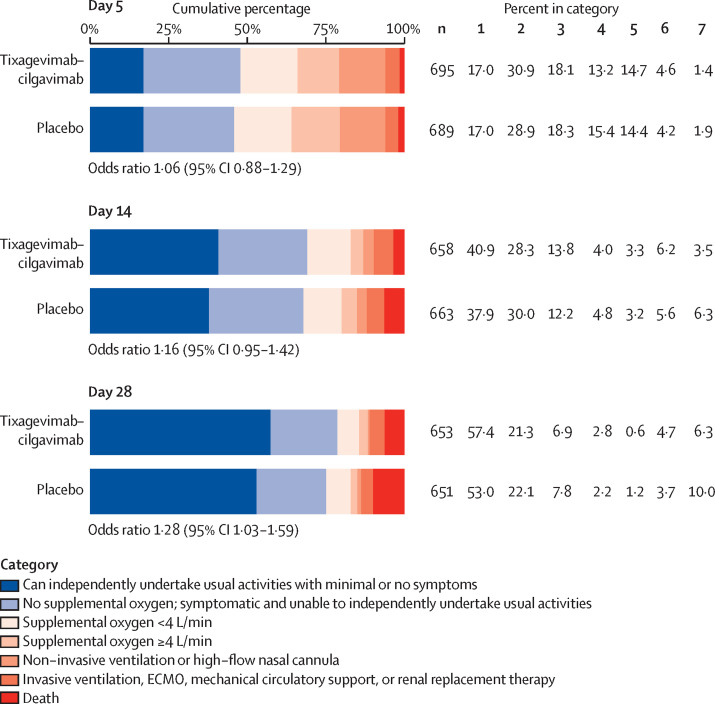
The proportion of participants across the pulmonary ordinal scale categories was not statistically different between treatment groups at day 5 (odds ratio [OR] 1·06 [95% CI 0·88–1·29]; p=0·52) and day 14 (OR 1·16 [0·95–1·42]; p=0·15), but favoured tixagevimab–cilgavimab at day 28 (OR 1·28 [1·03–1·59]; p=0·024; figure 4 ).
Figure 4.
Pulmonary ordinal scale at days 5, 14, and 28 for the full cohort
ECMO=extracorporeal membrane oxygenation. OR=odds ratio.
Tixagevimab–cilgavimab was generally well tolerated with low rates of treatment-emergent adverse events (appendix pp 43–53). Infusion reactions were relatively uncommon and similar in the tixagevimab–cilgavimab (41 [6%]) and placebo (48 [7%]) groups and were mostly of grade 1 or 2 in severity (appendix pp 43, 44).
The main safety outcome (composite of death, serious adverse events, incident organ failure, and serious co-infection through day 90) occurred in 178 (25%) participants in the tixagevimab–cilgavimab group and 212 (30%) in the placebo group (HR 0·83 [95% CI 0·68−1·01]; p=0·059; table 2, appendix pp 54−55).
Serious adverse events occurred in 34 (5%) participants in the tixagevimab−cilgavimab group and 38 (5%) in the placebo group (table 2, appendix pp 49−53). Most safety events were classified as respiratory−thoracic−mediastinal, gastrointestinal, nervous system, or general system organ classification. A similar proportion of participants in both groups experienced cardiovascular, thromboembolic, and renal adverse events.
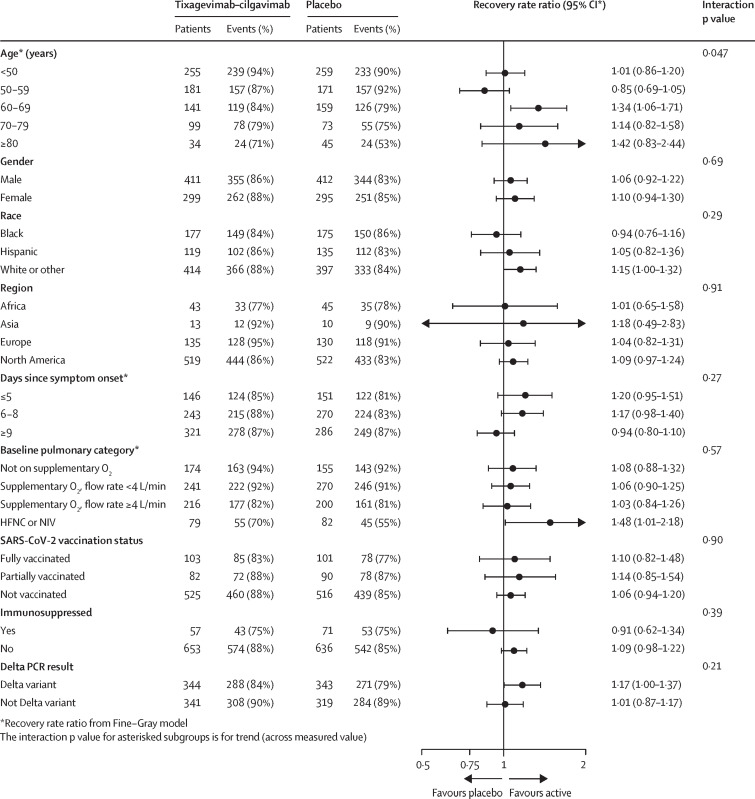
The presence of anti-SARS-CoV-2 antibodies at baseline measured by three assays (two anti-spike, one anti-nucleocapsid) did not substantively modify the effect of tixagevimab−cilgavimab on time to sustained recovery, death, or the composite safety outcome (appendix pp 45−48, 58−64). Since SARS-CoV-2 vaccination might influence baseline serostatus, we did a post-hoc analysis for unvaccinated participants only; results stratified by baseline serostatus were similar (appendix pp 43, 66). The treatment effect also did not vary substantially by geographical region, SARS-CoV-2 vaccination status, or immunocompromised state (appendix pp 54−62; figure 5 ). For sustained recovery and deaths up to day 90, point estimates of RRRs and HRs favoured earlier treatment with tixagevimab−cilgavimab for those within 9 days of symptom onset, participants infected with the delta variant, and those receiving high-flow nasal oxygen or non-invasive ventilation at baseline (figure 5, appendix pp 56−67).
Figure 5.
Subgroup analysis for time to sustained recovery up to day 90 for the full cohort
Continuous variables interaction p values are based on them being fitted as continuous rather than categorical. For baseline pulmonary category, participants were eligible for enrolment in the HFNC or NIV group, only after July 19, 2021; this corresponds to a pandemic phase when the delta variant was predominant. HFNC=high-flow nasal cannula. NIV=non-invasive ventilation. RRR=recovery rate ratio.
Discussion
In this multinational phase 3 trial involving patients hospitalised with COVID-19, treatment with a single infusion of tixagevimab−cilgavimab, compared with placebo, did not improve the primary outcome of time to sustained recovery, but led to a 30% relative risk reduction for mortality up to day 90. Absolute risk reduction was 3·6%. In contrast to earlier results with other monoclonal antibody treatments that showed more favourable results for seronegative than seropositive patients,7, 8, 9 results for tixagevimab−cilgavimab did not differ by baseline serostatus. There was also no evidence of harm either overall or by baseline serostatus. Taken together, these results suggest that tixagevimab−cilgavimab is safe and might reduce mortality among patients hospitalised with COVID-19. The reason for different results for sustained recovery and mortality might be related to the relatively high proportion of participants receiving no or low (<4 L/min) supplemental oxygen (59%), who tended to recover quickly with remdesivir and other standard care, or might be due to chance.
Outcomes with tixagevimab−cilgavimab differ from previous monoclonal antibodies in several ways. Tixagevimab−cilgavimab is the first in the ACTIV-3 platform that the DSMB recommended to proceed to full enrolment after early futility assessment and also the first to ultimately suggest lower mortality. Whereas two of the three agents previously studied were associated with possible harm in patients who are seropositive,8, 9 as was casirivimab−imdevimab in the RECOVERY trial but not in a company sponsored trial,7, 28 a safety signal was not observed with tixagevimab−cilgavimab in this subgroup, despite an approximately 90-day half-life that is longer than previous monoclonal antibodies.17 It is unknown whether this different result is attributable to the specific epitopes targeted by tixagevimab−cilgavimab or the Fc modifications that might reduce potential risk of antibody dependent enhancement of disease,29 versus changes over time in patients (such as vaccination), viral variants, or other epidemiological factors. More generally, these discordant results suggest that potential harm in patients who are seropositive is not a class-wide effect among all monoclonal antibodies.
As with other antiviral therapies, treatment earlier in the COVID-19 disease course might be more likely to be effective. Several monoclonal antibody treatments have shown benefit in reducing progression of disease when administered to outpatients in the first week of illness.1, 2, 3 In the present trial, almost all participants still had detectable viral antigen concentrations at baseline (median 8 days of symptoms), and nearly half did not have endogenous neutralising antibodies, suggesting that antivirals might remain an important component of the treatment approach in patients who are hospitalised. This result aligns with the first stage of the Adaptive COVID-19 Treatment Trial,30 in which treatment with the antiviral remdesivir was beneficial in patients enrolled at a median of 9 days, as well as with the RECOVERY trial, in which casirivemab−imdevimab showed a mortality reduction in patients who had been hospitalised who were seronegative at a median of 7 days of symptoms.7 Additionally, tixagevimab−cilgavimab was evaluated with concomitant remdesivir, in contrast to the RECOVERY trial in which only approximately 25% of patients received remdesivir. Although there are conflicting guidelines regarding its use for patients hospitalised with COVID-19, concomitant remdesivir treatment in this trial ensures that any observed benefits of tixagevimab−cilgavimab are additive to this background antiviral therapy. In RECOVERY, 28-day mortality was 20% compared with 10% in the current study. The results from the present trial suggest that monoclonal antibodies might provide clinical benefit in patients irrespective of whether they are receiving a direct-acting antiviral agent. As this benefit was not apparent with the pulmonary ordinal scale at day 5 (used for early futility assessment in all TICO trials), future clinical trials in inpatients with COVID-19 might be best served by assessing early futility by means of a later timepoint, a different outcome, or both.
Strengths of this trial include a large, diverse patient population enrolled at experienced sites on four continents. Additionally, there was blinded administration of the investigational agent, detailed attention to outcome ascertainment, and continuous review from the DSMB. Although ACTIV-3/TICO is a multi-arm platform, patients were randomly assigned to only one investigational agent and contemporaneous placebos were used, so the results are not influenced by other investigational agents. Although underpowered for subgroup analyses, there was no compelling evidence of differences between tixagevimab−cilgavimab and placebo in either efficacy or safety by baseline serostatus, region, comorbid conditions, including immunocompromised state, or by vaccination status, suggesting that the results are broadly generalisable. The potential for benefit in patients receiving high-flow nasal cannula or mechanical ventilation merits further study.
Our trial has several limitations. First, enrolment concluded before the emergence of the omicron variant, so we do not have direct evidence for patients infected with this or future variants. However, in vitro data suggest that although the half maximal inhibitory concentration (IC50) for tixagevimab−cilgavimab is higher against the omicron (B.1.1.529 or BA.2) variant as compared with previous variants,31, 32, 33, 34 clinical activity might be retained, albeit higher doses might be required.4, 13, 14 Second, only a minority of participants were fully vaccinated, making it difficult to extrapolate our results to vaccinated or boosted patients. Third, critically ill patients already requiring mechanical ventilation, vasopressors, or new renal replacement therapy were excluded. Reassuringly, nominal differences for efficacy and safety favoured tixagevimab−cilgavimab for participants receiving high-flow nasal cannula or non-invasive ventilation at baseline. Fourth, since almost all patients received remdesivir per protocol, the benefit of tixagevimab−cilgavimab if used as antiviral monotherapy is unknown. Broad adherence to guideline-recommended treatment, including remdesivir and corticosteroids, might reduce the potential for larger effect sizes, and the study had limited power to detect a modest treatment effect for sustained recovery. Yet, the observed mortality benefit highlights the ongoing need for further rescue interventions for patients hospitalised with COVID-19.
A single infusion of tixagevimab−cilgavimab, added to background therapy with remdesivir and other standard care, did not reduce time to the primary outcome of sustained recovery among patients hospitalised with COVID-19. However, this combination monoclonal antibody was safe and resulted in a 30% lower mortality than standard care alone, suggesting that tixagevimab−cilgavimab might be a useful treatment option for patients hospitalised with COVID-19.
Correspondence to: Prof Adit A Ginde, University of Colorado School of Medicine, Aurora, CO 80045, USA adit.ginde@cuanschutz.edu
Data sharing
Deidentified data from TICO trials will be made available 1 year after publication of final results from the platform. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted by means of the research proposal form on the INSIGHT website.
Declaration of interests
TLH reports consulting fees from Lysovant, royalties for UpToDate topic authorship, and participation on a Staphylococcus Aureus Network Adaptive Platform Trial Data and Safety Monitoring Board (DSMB), outside of the submitted work. AAG reports grants from US National Institutes of Health (NIH) during the conduct of the study, grants from US Centers for Disease Control (CDC), US Department of Defense (DOD), AbbVie, and Faron Pharmaceuticals, and participation on a NIH DSMB, outside of the submitted work. RP reports grants from Gilead, ViiV, and MSD and consulting fees from Gilead, ViiV, MSD, Theratechnologies, and Eli Lilly, outside of the submitted work. TAM reports grants from National Institute of Allergies and Infectious Diseases (NIAID), NIH, and Leidos, outside of the submitted work. GG reports partial salary support from NIH through the University of Minnesota during the conduct of the study. US reports grants from ViiV, Cytodyn, NIAID, and Cardiothoracic Surgical Trial Network, consulting fees from ViiV and Shionogi, and payment for educational events from Paratek and Shionogi, outside of the submitted work. RLG reports contracts from Regeneron, consulting fees from Gilead and GSK Pharmaceuticals, participation on advisory boards for Eli Lilly, Gilead, GSK, Johnson and Johnson, Roche–Genentech, and Kinevant Sciences, de minimis stock in AbCellera, and receipt of medication from Gilead as an in-kind gift to facilitate the conduct of an academic-sponsored clinical trial, outside of the submitted work. MKJ reports grants from Gilead, participation on an advisory board for Gilead, and a Board of Director position with the HIV Medication Association, outside of the submitted work. LM reports payment for educational events from Merck, travel support from Merck and Gilead, and participation on an advisory board for Merck, outside of the submitted work. EM reports grants from NIAID and NIH, outside of the submitted work. KK reports grants and contracts with NIH, Regeneron, Abbott, Pfizer, Romark, and Raisonance, consulting fees from Regeneron, travel support from Sanford Guide and Burroughs Wellcome Fund, a patent pending for 3D swabs, and leadership positions at Sanford Guide and Burroughs Wellcome Fund, outside of the submitted work. PEM reports grants from NHLBI and NIH during the conduct of the study, grants and contracts from NHLBI and NIH, and consulting fees from Dompe, Medtronic, and Boerhinger Ingelheim, outside of the submitted work. BT reports grants from VA Cooperative Studies Program Coordinating Center during the conduct of the study and grants and consulting fees from Genentech, outside of the study. KUK reports grants from NIH during the conduct of the study. MAM reports grants from NIH, DOD, the California Institute of Regenerative Medicine, Roche-Genentech, and Quantum Therapeutics and consulting fees from Novartis, Johnson and Johnson, Citius Pharmaceuticals, Pilant Therapeutics, and Gilead, outside of the submitted work. GP reports consulting fees from Menarini, Pfizer, MSD, and Gilead, payment for education events from Pfizer, MSD, AstraZeneca, and Gilead, and participation on advisory boards for Pfizer, Gilead, and MSD, outside of the submitted work. KNS reports consulting fees from Roche, Bristol Myers Squibb (BMS), Amgen, and MSD, outside of the submitted work. SRB reports consulting fees from Gilead, MSD, and GSK, payment for educational events from Gilead and MSD, and participation on advisory boards for Gilead, MSD, Pfizer, Roche, and ViiV, outside of the submitted work. JDC reports grants from NIH, outside of the submitted work. PC reports grants from NIH during the conduct of the study and grants from Eli Lilly, Regeneron, and Gilead, consulting fees from Eli Lilly, Regeneron, and Gilead, and payment for education events from Frontier Collaborative, Physician Education Resource, Rockpointe, CME Outfitter, outside of the submitted work. DJD reports grants from NIH during the conduct of the study. DCF reports grants from NIH during the conduct of the study, grants from NIH and CDC, consulting fees from Cytovale, and participation on a DSMB for Medpace, outside of the submitted work. HFG reports grants from the Swiss National Science Foundation, Swiss HIV Cohort Study, Gilead, the Yvonne Jacob Foundation, and NIH and participation on advisory boards for Merck, Gilead, ViiV, Janssen, and Novartis, outside of the submitted work. RDH reports grants from NHLBI, Airway Therapeutics, Incyte Corporation, and Kiniksa Pharmaceuticals and participation on a NIH DSMB outside of the submitted work. AK reports grants from United Therapeutics, Johnson & Johnson, Eli Lilly, AstraZeneca, and 4DMedical, outside of the submitted work. JSO reports grants from NIH during the conduct of the study. JSS reports grants from NIH during the conduct of the study. BEY reports consulting fees from Gilead and Novacyte and payment for educational events from AstraZeneca, Gilead, Sanofi, and Roche, outside of the submitted work. ANP reports grants from Bill and Melinda Gates Foundation (BMGF), UK Research and Innovation (UKRI), the Wellcome Trust, and the National Institute for Health and Care Research (NIHR) and consulting fees from BMGF, outside of the submitted work. DDM reports support from DNRF126 during the conduct of the study. MLP reports grants from Boehringer-Ingelheim, payment for authorship from Genentech and France Foundation, and travel support from Eastern Pulmonary Conference, outside of the submitted work. DS reports salary support from NIH through Axle Informatics during the conduct of the study and a leadership position for EigenMed, outside of the submitted work. VN reports grants from NIH during the conduct of the study. SLP reports grants from the University of Minnesota during the conduct of the study and grants from Gilead, ViiV, Janssen, the European and Developing Countries Clinical Trials Partnership, the Medical Reserve Corps (MRC), and NIHR, and participation on a NIHR DSMB, outside of the submitted work. GT reports grants from University College London, outside of the submitted work. SMB reports grants from NIH during the conduct of the study and grants from NIH and participation on a DSMB for Hamilton, outside of the submitted work. WHS reports grants from NIH and NHLBI during the conduct of the study. BG reports grants from NIH during the conduct of the study. SS reports grants from NIH, outside of the conduct of the study. CSR reports grants from NIH during the conduct of the study. PG reports being an employee of AstraZeneca, being a full member of Academia de Medicina, Rio de Janeiro, Brazil, and stock ownership at Takeda. MTE reports stock ownership and being an employee at AstraZeneca. AT reports and stock ownership and being an employee at AstraZeneca. AGB reports grants from University of Minnesota, MRC, and UKRI during the conduct of the study. VJD reports grants from NIAID–NIH and a contract with the University of Minnesota, outside of the conduct of the study. GM reports grants from Gilead, AbbVie, and Viiv, payment for a speaking engagement from Janssen, participation on advisory boards for AstraZeneca and Gilead, and a leadership role on the Australian National COVID-19 taskforce treatments committee, outside of the conduct of the study. BTT reports grants from NHLBI and consulting fees from Bayer, Novartis, and Thetis, outside of the conduct of the study. JDN reports grants from NIH and NIAID during the conduct of the study. All other members of the writing committee declare no competing interests.
Acknowledgments
Acknowledgments
We thank the members of the ACTIV-3–TICO data and safety monitoring board for their review of the protocol and their guidance based on interim reviews of the data. We also thank the participants and families whose dedication to contributing to science made this research study possible. The Division of Clinical Research at the National Institute of Allergy and Infectious Diseases funded this project. Investigators from NIH were directly involved in all aspects of this study, including study design, data collection, data analysis, data interpretation, and writing of the report. All analyses of biological material were done in a masked manner at laboratories affiliated with the funding source; data were sent to the statistical and data management center at the University of Minnesota for linkage to the trial database. Several representatives from the funding source are part of the writing group for the manuscript. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing official policies, either expressed or implied, of the National Institutes of Health or the Department of Veterans Affairs. Supported by the US Operation Warp Speed program, the National Institute of Allergy and Infectious Diseases and Leidos Biomedical Research for the INSIGHT Network, the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the Prevention and Early Treatment of Acute Lung Injury Network and the Cardiothoracic Surgical Trials Network, the US Department of Veterans Affairs, and grants from the governments of Denmark, Australia, the UK, and Singapore. The research was, in part, funded by National Institutes of Health Agreement 1OT2HL156812-01 and National Cancer Institute contract 75N91019D00024, task order number 75N91020F00039. Trial medications were donated by AstraZeneca (tixagevimab/cilgavimab) and Gilead Sciences (remdesivir).
ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group
Writing group: Thomas L Holland*, Adit A Ginde*, Roger Paredes*, Thomas A. Murray, Nicole Engen, Greg Grandits, Andrew Vekstein, Noel Ivey, Ahmad Mourad, Uriel Sandkovsky, Robert L Gottlieb, Mezgebe Berhe, Mamta K Jain, Rubria Marines-Price, Barbine Tchamba Agbor Agbor, Lourdes Mateu, Sergio España-Cueto, Gemma Lladós, Eleftherios Mylonakis, Ralph Rogers, Fadi Shehadeh, Michael R Filbin, Kathryn A Hibbert, Kami Kim, Thanh Tran, Peter E Morris, Evan P Cassity, Barbara Trautner, Lavannya M Pandit, Kirk U Knowlton, Lindsay Leither, Michael A Matthay, Angela J Rogers, Wonder Drake, Beatrice Jones, Garyfallia Poulakou, Konstantinos N Syrigos, Eduardo Fernández-Cruz, Marisa Di Natale, Eyad Almasri, Leire Balerdi-Sarasola, Sanjay R Bhagani, Katherine L Boyle, Jonathan D Casey, Peter Chen, David J Douin, D Clark Files, Huldrych F Günthard, R Duncan Hite, Robert C Hyzy, Akram Khan, Moses Kibirige, Robert Kidega, Ivan Kimuli, Francis Kiweewa, Jens-Ulrik Jensen, Bradley G Leshnower, Joseph K Lutaakome, Prasad Manian, Jose Luis Morales-Rull, D Shaney O'Mahony, J Scott Overcash, Srikant Ramachandruni, Jay S Steingrub, Hassan S Taha, Michael Waters, Barnaby E Young, Andrew N Phillips, Daniel D Murray, Tomas O Jensen, Maria L Padilla, David Sahner, Katy Shaw-Saliba, Robin L Dewar, Marc Teitelbaum, Ven Natarajan, M Tauseef Rehman, Sarah Pett, Fleur Hudson, Giota Touloumi, Samuel M Brown, Wesley H Self, Christina C Chang, Adriana Sánchez, Amy C Weintrob, Timothy Hatlen, Birgit Grund, Shweta Sharma, Cavan S Reilly, Pedro Garbes, Mark T Esser, Alison Templeton, Abdel G Babiker, Victoria J Davey, Annetine C Gelijns, Elizabeth S Higgs, Virginia Kan, Gail Matthews, B Taylor Thompson, James D Neaton, Clifford Lane, Jens D Lundgren *Contributed equally
Affiliations
Division of Infectious Diseases, Duke University, Durham, North Carolina, USA (T L Holland); Department of Emergency Medicine, University of Colorado School of Medicine; Aurora, Colorado, USA (Prof A A Ginde MD); Infectious Diseases Department & irsiCaixa AIDS Research Institute, Hospital Universitari Germans Trias i Pujol, Catalonia, Spain (Prof R Paredes MD); Division of Biostatistics, School of Public Health, University of Minnesota; Minneapolis, Minnesota, USA (T A Murray PhD, N Engen MS, G Grandits MS); Division of Cardiovascular and Thoracic Surgery, Department of Surgery, Duke University Medical Center, Durham, NC, USA (A Vekstein MD); Department of Medicine (N Ivey MD), Division of Infectious Diseases (A Mourad MD), Duke University Medical Center, Durham, NC, USA; Division of Infectious Diseases, Baylor University Medical Center, Dallas, TX, USA (U Sandkovsky MD); Center for Advanced Heart and Lung Disease, Baylor University Medical Center; Dallas, TX, USA. Baylor Heart and Vascular Hospital; Dallas, TX, USA. Baylor Scott and White, The Heart Hospital—Plano; Plano, TX, USA (R L Gottlieb PhD); Baylor University Medical Center; Dallas, TX, USA (M Berhe MD); Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, UT Southwestern Medical Center and Parkland Health & Hospital System; Dallas, TX, USA (Prof M K Jain MD); Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, UT Southwestern Medical Center and Parkland Health & Hospital System; Dallas, TX, USA (R Marines-Price PhD); Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, UT Southwestern Medical Center and Parkland Health & Hospital System; Dallas, TX, USA (B T Agbor Agbor MD); Infectious Diseases Department, Hospital Universitari Germans Trias i Pujol, Catalonia, Spain (L Mateu PhD); Infectious Diseases Department, Hospital Universitari Germans Trias i Pujol, Catalonia, Spain (S España-Cueto MD); Infectious Diseases Department, Hospital Universitari Germans Trias i Pujol, Catalonia, Spain (G Lladós MD); Division of Infectious Diseases, Rhode Island Hospital & The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island, USA (Prof Eleftherios Mylonakis MD); Division of Infectious Diseases, Rhode Island Hospital & The Miriam Hospital, Alpert Medical School of Brown University, Providence, RI, USA (R Rogers MD); Division of Infectious Diseases, Rhode Island Hospital, Providence, RI, USA (F Shehadeh MSc); Department of Emergency Medicine, Massachusetts General Hospital, Boston, MA, USA (M R Filbin MD); Division of Pulmonary and Critical Care Medicine, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA (K A Hibbert MD); Division of Infectious Disease and International Medicine, University of South Florida, Global Emerging Diseases Institute, Tampa General Hospital, Tampa, Florida, USA (Prof K Kim MD); Division of Infectious Disease and International Medicine, University of South Florida, Tampa, Florida, USA (T Tran MPH); University of Kentucky, Lexington, Kentucky, USA (Prof P E Morris MD); University of Kentucky, Lexington, Kentucky, USA (E P Cassity MD); Michael E. DeBakey Veterans Affairs Medical Center/Baylor College of Medicine, Houston, Texas, USA (Prof B Trautner MD); Michael E DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, Houston, TX, USA (L M Pandit MD); Cardiovascular Department, Intermountain Medical Center, Salt Lake City, UT, USA (Prof K U Knowlton MD); Intermountain Medical Center, Salt Lake City, UT, USA (L Leither DO); Cardiovascular Research Institute, Departments of Medicine and Anesthesia, University of California, San Francisco, CA, USA (Prof M A Matthay MD); Division of Pulmonary, Allergy, and Critical Care Medicine, Stanford University, Stanford, California, USA (A J Rogers MD); VA Tennessee Valley Healthcare System Nashville Campus, Vanderbilt University School of Medicine, Nashville, Tennessee, USA (Prof W Drake MD); VA Tennessee Valley Healthcare System Nashville Campus, Nashville, TN, USA (B Jones RN); Sotiria General Hospital, National and Kapodistrian University of Athens, Athens, Greece (G Poulakou MD); Sotiria General Hospital, National and Kapodistrian University of Athens, Athens, Greece (K N Syrigos MD); Department of Inmunología Clínica. Hospital General Universitario Gregorio Marañon. Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain (E Fernández-Cruz MD); Department of Inmunología Clínica. Hospital General Universitario Gregorio Marañon. Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain (M Di Natale MD); Pulmonary and Critical Care Division, University of California San Francisco, Fresno MEP, Fresno, California, USA (E Almasri MD); Hospital Clinic Barcelona- IS Global, Barcelona, Spain (L Balerdi-Sarasola MD); Royal Free Hospital NHS Foundation Trust, London, UK (S R Bhagani MD); Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA (K L Boyle MD); Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center; Nashville, Tennessee, USA (J D Casey MD); Division of Pulmonary and Critical Care Medicine, Cedars-Sinai Medical Center, Los Angeles, CA USA (Prof P Chen MD); Department of Anesthesiology, University of Colorado School of Medicine, Aurora, CO, USA (D J Douin MD); Section on Pulmonary, Critical Care, Allergy and Immunologic Disease, Wake Forest Baptist Health; Winston-Salem, NC, USA (D Clark Files MD); Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich; Zurich and Institute of Medical Virology, University of Zurich; Zurich, Switzerland (Prof H F Günthard MD); Division of Pulmonary, Critical Care and Sleep Medicine, University of Cincinnati College of Medicine; Cincinnati, OH, USA (Prof R D Hite MD); Division of Pulmonary and Critical Care, University of Michigan, Ann Arbor, Michigan, USA (Prof R C Hyzy MD); Division of Pulmonary and Critical Care Medicine, Oregon Health & Science University; Portland, Oregon, USA (A Khan MD); Masaka Regional Referral Hospital, Masaka, Uganda (M Kibirige MBChB); Gulu Regional Referral Hospital, Gulu, Uganda (R Kidega MBChB); Makerere University Lung Institute, Kampala, Uganda (I Kimuli MBChB); Strengthening Institutional Capacity for Research Administration (SICRA); and Lira Regional Referral Hospital, Kampala, Uganda (F Kiweewa MBChB); Section of Respiratory Medicine, Department of Medicine Herley-Gentofte Hospital, Hellerup, Denmark; CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet; Department of Clinical Medicine, Faculty of Health Sciences, University of Copenhagen; Copenhagen, Denmark (Prof J-U Jensen MD); Division of Cardiothoracic Surgery, Department of Surgery, Emory University, Atlanta, Georgia, USA (B G Leshnower MD); Medical Research Council/ Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe; St Francis Hospital, Nsambya, Kampala, Uganda (J K Lutaakome MD); Texas Heart Institute, Baylor College of Medicine, Houston, TX, USA (Prof P Manian MD); New York City Health and Hospitals, Lincoln, Bronx, NY, USA (V Menon MD); Internal Medicine Department. University Hospital Arnau de Vilanova, Lleida, Spain (J L Morales-Rull MD); Swedish Medical Center, Seattle, WA, USA (D S O'Mahony MD); Velocity, San Diego, CA, USA (J S Overcash MD); Christus Spohn Health System, Corpus Christi, TX, USA (S Ramachandruni MD); Division of Pulmonary and Critical Care, Baystate Medical Center, Springfield, MA, USA (Prof J S Steingrub MD); Cotton O'Neil, Topeka, Kansas, USA (H S Taha MD); Velocity, Chula Vista, CA, USA (M Waters MD); National Centre for Infectious Diseases, Singapore (B E Young MD); Institute for Global Health, University College London, UK (Prof A N Phillips PhD); CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet; Copenhagen, Denmark (D D Murray PhD); CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark; North Zealand University Hospital, Department of Pulmonary and Infectious Diseases, Hillerod, Denmark (T O Jensen MD); Mount Sinai School of Medicine, New York, NY, USA (Prof M L Padilla MD); Axle Informatics, Rockville, MD, USA (D Sahner MD); National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA (K Shaw-Saliba PhD); Frederick National Laboratory for Cancer Research, Frederick, MD, USA (R L Dewar PhD); Leidos Biomedical Research, Frederick, MD, USA (M Teitelbaum MD); Frederick National Laboratory for Cancer Research, Frederick, MD, USA (V Natarajan PhD, M Tauseef Rehman MA); The Medical Research Council Clinical Trials Unit at UCL, University College London; London, UK (Prof S Pett MD, F Hudson); National and Kapodistrian University of Athens, Athens, Greece (Prof G Touloumi PhD); Division of Pulmonary and Critical Care Medicine, Intermountain Medical Center; Department of Internal Medicine, University of Utah; Murray, UT, USA (Prof S M Brown MD); Department of Emergency Medicine, Vanderbilt University Medical Center; Nashville, Tennessee, USA (W H Self MD); The Kirby Institute, University of New South Wales; Sydney, Australia (C C Chang MD); Washington DC VA Medical Center Washington DC, USA (A Sánchez MS); Infectious Diseases Section, Washington, DC VA Medical Center, Washington DC, USA (A C Weintrob MD); Lundquist Institute and Harbor-UCLA Medical Center, Torrance, CA, USA (T Hatlen MD); School of Statistics, University of Minnesota; Minneapolis, Minnesota, USA (Prof B Grund PhD); Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, USA (S Sharma MS, Prof C S Reilly PhD); AstraZeneca, Gaithersburg, MD, USA (P Garbes MD, M T Esser PhD); AstraZeneca, Cambridge, UK (A Templeton PhD); The Medical Research Council Clinical Trials Unit at UCL, University College London, London, UK (A G Babiker PhD); United States Department of Veterans Affairs, Washington DC, USA (V J Davey PhD); Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA (Prof A C Gelijns PhD); National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA (E S Higgs MD); Infectious Diseases Section, VA Medical Center, Washington DC, USA (Prof V Kan MD); The Kirby Institute, University of New South Wales; Sydney, Australia (Prof G Matthews MRCP); Division of Pulmonary and Critical Care, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA (Prof B Taylor Thompson MD); Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, USA (Prof J D Neaton PhD); National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA (H C Lane MD); CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark (Prof J D Lundgren MD)
Contributors
JDN, TAM, NE, and GG directly accessed and verified the underlying data. JDL, HCL, JDN, AGB, VJD, ACG, ESH, VK, and BTT were responsible for conceptualisation. All authors were responsible for the investigation and reviewing and editing the manuscript. JDN, TAM, NE, and GG were responsible for data curation. TAM, NE, and GG were responsible for formal analysis JDN, AGB, VJD, ACG, VK, BTT, and JDL were responsible for funding acquisition. JDL, HCL, JDN, AGB, VJD, ACG, ESH, VK, GM, and BTT were responsible for supervision. TLH, AAG, and RP composed the initial manuscript.
Contributor Information
ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group:
Thomas L. Holland, Adit A. Ginde, Roger Paredes, Thomas A. Murray, Nicole Engen, Greg Grandits, Andrew Vekstein, Noel Ivey, Ahmad Mourad, Uriel Sandkovsky, Robert L. Gottlieb, Mezgebe Berhe, Mamta K. Jain, Rubria Marines-Price, Barbine Tchamba Agbor Agbor, Lourdes Mateu, Sergio España-Cueto, Gemma Lladós, Eleftherios Mylonakis, Ralph Rogers, Fadi Shehadeh, Michael R. Filbin, Kathryn A. Hibbert, Kami Kim, Thanh Tran, Peter E. Morris, Evan P. Cassity, Barbara Trautner, Lavannya M. Pandit, Kirk U. Knowlton, Lindsay Leither, Michael A. Matthay, Angela J. Rogers, Wonder Drake, Beatrice Jones, Garyfallia Poulakou, Konstantinos N. Syrigos, Eduardo Fernández-Cruz, Marisa Di Natale, Eyad Almasri, Leire Balerdi-Sarasola, Sanjay R. Bhagani, Katherine L. Boyle, Jonathan D. Casey, Peter Chen, David J. Douin, D. Clark Files, Huldrych F. Günthard, R. Duncan Hite, Robert C. Hyzy, Akram Khan, Moses Kibirige, Robert Kidega, Ivan Kimuli, Francis Kiweewa, Jens-Ulrik Jensen, Bradley G. Leshnower, Joseph K. Lutaakome, Prasad Manian, Vidya Menon, Jose Luis Morales-Rull, D. Shane O'Mahony, J. Scott Overcash, Srikant Ramachandruni, Jay S. Steingrub, Hassan S. Taha, Michael Waters, Barnaby E. Young, Andrew N. Phillips, Daniel D. Murray, Tomas O. Jensen, Maria L. Padilla, David Sahner, Katy Shaw-Saliba, Robin L. Dewar, Marc Teitelbaum, Ven Natarajan, M. Tauseef Rehman, Sarah Pett, Fleur Hudson, Giota Touloumi, Samuel M. Brown, Wesley H. Self, Christina C. Chang, Adriana Sánchez, Amy C. Weintrob, Timothy Hatlen, Birgit Grund, Shweta Sharma, Cavan S. Reilly, Pedro Garbes, Mark T. Esser, Alison Templeton, Abdel G. Babiker, Victoria J. Davey, Annetine C. Gelijns, Elizabeth S. Higgs, Virginia Kan, Gail Matthews, B. Taylor Thompson, James D. Neaton, H. Clifford Lane, and Jens D. Lundgren
Supplementary Material
References
- 1.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV Antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 3.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration . US Food and Drug Administration; Silver Spring, MD: 2022. Letter of authorization for emergency use of EVUSHELD (tixagevimab and cilgavimab)https://www.fda.gov/media/154704/download [Google Scholar]
- 5.O'Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Nirula A, Mulligan MJ, et al. Effect of Bamlanivimab vs Placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46–55. doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abani O, Abbas A, Abbas F, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren JD, Grund B, Barkauskas CE, et al. Responses to a neutralizing monoclonal antibody for hospitalized patients with covid-19 according to baseline antibody and antigen levels: a randomized controlled trial. Ann Intern Med. 2022;175:234–243. doi: 10.7326/M21-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22:622–635. doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28:490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. N Engl J Med. 2022;386:995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Statement Coronavirus (COVID-19) Update: FDA Limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. January 24, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron
- 13.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rockett R, Basile K, Maddocks S, et al. Resistance mutations in SARS-CoV-2 delta variant after sotrovimab use. N Engl J Med. 2022;386:1477–1479. doi: 10.1056/NEJMc2120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J, Zost SJ, Greaney AJ, et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat Microbiol. 2021;6:1233–1244. doi: 10.1038/s41564-021-00972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo YM, McTamney PM, Arends RH, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med. 2022 doi: 10.1126/scitranslmed.abl8124. published online Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oganesyan V, Damschroder MM, Woods RM, Cook KE, Wu H, Dall'acqua WF. Structural characterization of a human Fc fragment engineered for extended serum half-life. Mol Immunol. 2009;46:1750–1755. doi: 10.1016/j.molimm.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr. 2008;64:700–704. doi: 10.1107/S0907444908007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray DD, Babiker AG, Baker JV, et al. Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3) Clin Trials. 2022;19:52–61. doi: 10.1177/17407745211049829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 23.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 24.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67:661–670. doi: 10.1111/j.1541-0420.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176–182. doi: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]
- 28.Somersan-Karayaka S, Mylonakis E, Menon VP, et al. Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19. medRxiv. 2021 doi: 10.1101/2021.11.05.21265656. published online Jan 27. (prepr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junqueira C, Crespo A, Ranjbar S, et al. Fc-R-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606:576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 -- final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348. doi: 10.1016/j.cell.2021.02.037. 61.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939. doi: 10.1016/j.cell.2021.03.055. 54.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220. doi: 10.1016/j.cell.2021.06.020. 36.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruel T, Hadjadj J, Maes P, et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28:1297–1302. doi: 10.1038/s41591-022-01792-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from TICO trials will be made available 1 year after publication of final results from the platform. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted by means of the research proposal form on the INSIGHT website.