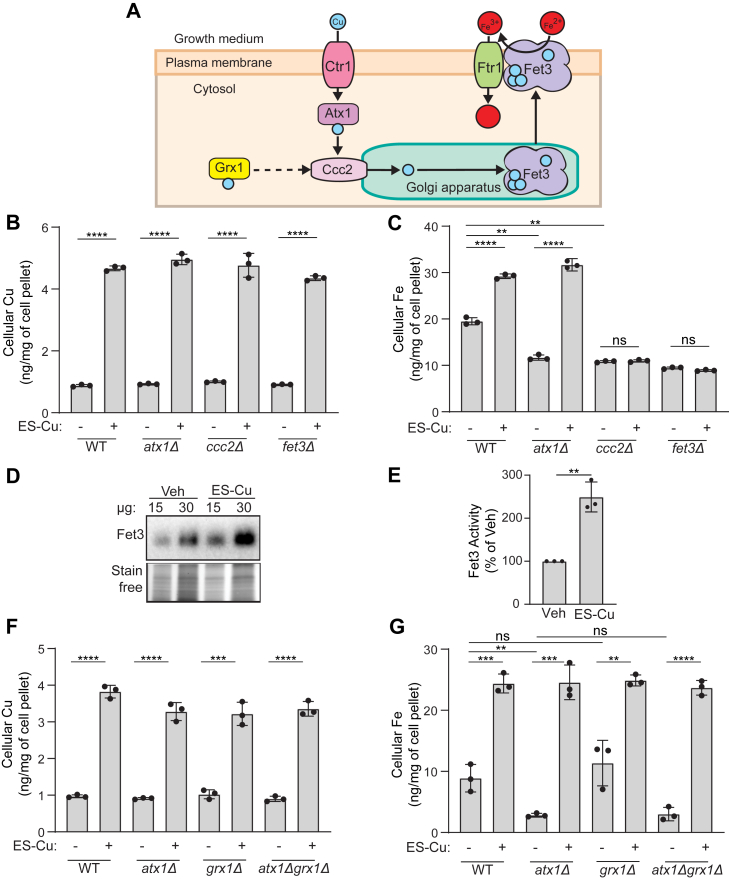
Figure 5.
ES-trafficked Cu is bioavailable to Ccc2 for its insertion into Fet3.A, a schematic of Cu transport to the Fe import machinery protein Fet3. Cu is imported via the high affinity Cu transporter Ctr1. Once inside the cell, Cu is bound by the Cu-metallochaperone Atx1, which transports Cu to Ccc2, a P-type ATPase present on the Golgi membrane that pumps Cu into the Golgi lumen where Fet3 metalation takes place. Metalated Fet3 is subsequently transported to the plasma membrane where it works in conjunction with Ftr1 to import extracellular Fe into cells. GRX1 has been suggested to transport Cu to Ccc2 homolog ATP7B, indicated by the dashed line. B and C, BY4741 WT, atx1Δ, ccc2Δ, and fet3Δ were grown in YPD ± 0.25 μM ES-Cu for 10 h before measuring Cu (B) and Fe (C) levels by ICP-MS. D, in-gel Fet3 oxidase activity assay performed on cellular extracts from vehicle or 0.25 μM ES-Cu–treated BY4741 WT cells. Stain-free imaging of the same gel was used as a loading control. E, quantification of Fet3 activity for 15 μg protein samples from panel (D). F and G, BY4741 WT, atx1Δ, grx1Δ, and atx1Δgrx1Δ cells were grown in YPD ± 0.25 μM ES-Cu for 10 h before measuring Cu (F) and Fe (G) levels by ICP-MS. Data are expressed as mean ± SD; (n = 3), ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, ns = not significant. ES, elesclomol; ES-Cu, elesclomol-copper complex; GRX1, glutaredoxin-1; ICP-MS, inductively coupled plasma-mass spectrometry.