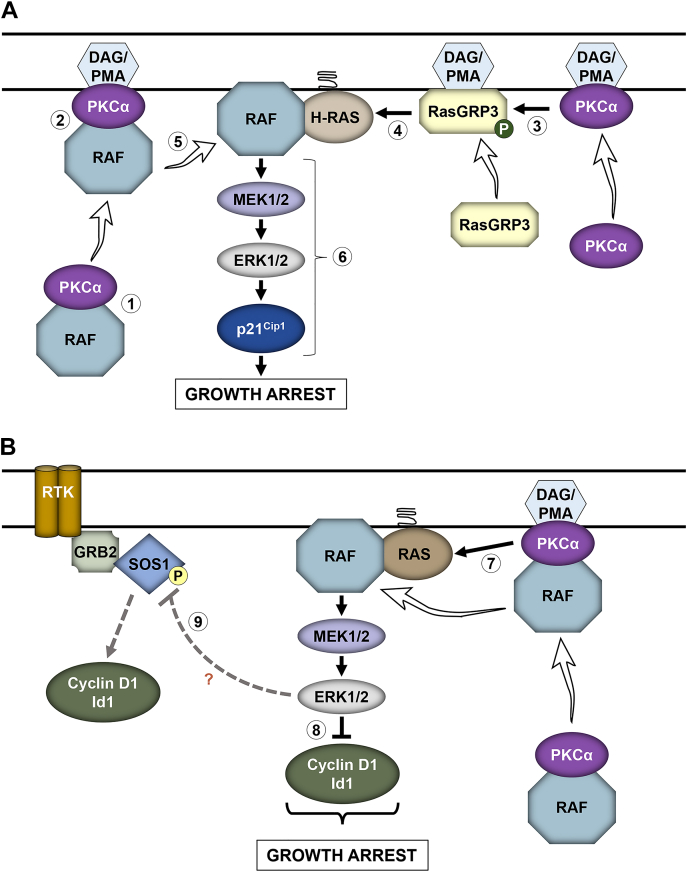
Figure 12.
Model for PKCα-induced ERK-dependent cell cycle arrest in the intestinal epithelium.A, PKCα-induced RasGRP3–H-Ras–ERK–p21Cip1 signaling. In the resting state, inactive PKCα associates with inactive Raf proteins in the cytoplasm (1); PKC agonists, such as PMA or DAG (generated by ligand induced hydrolysis of membrane phospholipids), recruit PKCα and associated Raf proteins to the plasma membrane (2); PMA/DAG also recruit RasGRP3 to the plasma membrane, where it is phosphorylated (P) and activated by membrane-localized PKCα (3); activated RasGRP3 promotes GTP-loading and activation of H-Ras (4); PKCα dissociates from Raf proteins, which can then interact with nearby activated Ras (5) for efficient activation of the ERK signaling pathway and induction of the cell cycle inhibitory molecule p21Cip1 (6) and growth arrest. B, PKCα-induced Ras–ERK–cyclin D1/Id1 signaling. PKCα also activates K- and/or N-Ras through at least one other undefined mechanism (7), leading to ERK-dependent downregulation of cyclin D1 and Id1 (8). PKCα also promotes ERK-dependent phosphorylation of SOS1 (9), which may negatively modulate SOS1 interaction with Grb2, Shc, or the EGF receptor to dampen growth factor signaling, thereby downregulating growth factor–induced cyclin D1 and Id1 and inhibiting proliferation. DAG, diacylglycerol; PMA, phorbol 12-myristate 13-acetate.