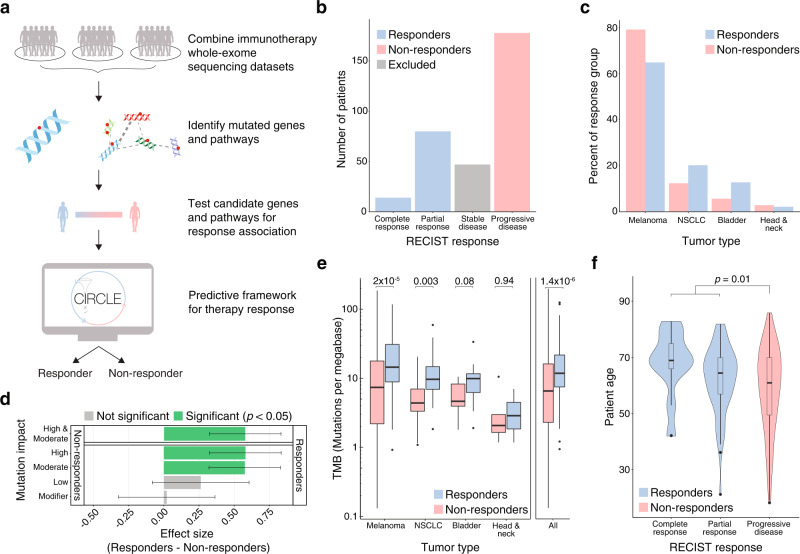
Fig. 1. An aggregated cohort of immune checkpoint blockade (ICB) patients replicates known correlations between tumor mutational burden and age with treatment response.
a Overview of the two-stage approach for immunotherapy response prediction. We pooled 6 cohorts of immune checkpoint blockade (ICB) recipients with matched whole-exome sequencing (WES) and Response Evaluation Criteria in Solid Tumors (RECIST) classification. We identified genes and pathways under positive selection and tested the nominated genes and pathways for their ability to predict ICB response. The significant predictors were used to develop and test an ICB response prediction algorithm. b Number of patients from the aggregated set of 6 cohorts in each RECIST response group. Patients with stable disease were excluded from analyses and the RECIST classifications of complete response and partial response were both considered responders. c Proportion of tumor types amongst ICB responders and non-responders. d Enrichment (effect size, Hedge’s g) for different types of mutations in responders (n = 94) and non-responders (n = 178) to ICB therapy. Error bars represent the 95% confidence interval and significance was determined using a two-sided Welch’s t test with Bonferroni correction. Tumor Mutational Burden (TMB) is the union of High and Moderate mutations. e TMB for responders (n = 94) and non-responders (n = 178) to ICB therapy by tumor type. Statistical significance was tested using two-tailed Welch’s t tests of log2 TMB. f Patient ages for different RECIST response groups (complete response n = 14, partial response n = 80, progressive disease n = 178). Statistical significance was tested using a two-tailed Welch’s t test. In e and f, the boxplot center line denotes median, with box limits being the 25th and 75th percentile. Boxplot whiskers indicate 1.5 times the interquartile range, while outliers above/below the whiskers are represented individually as points.