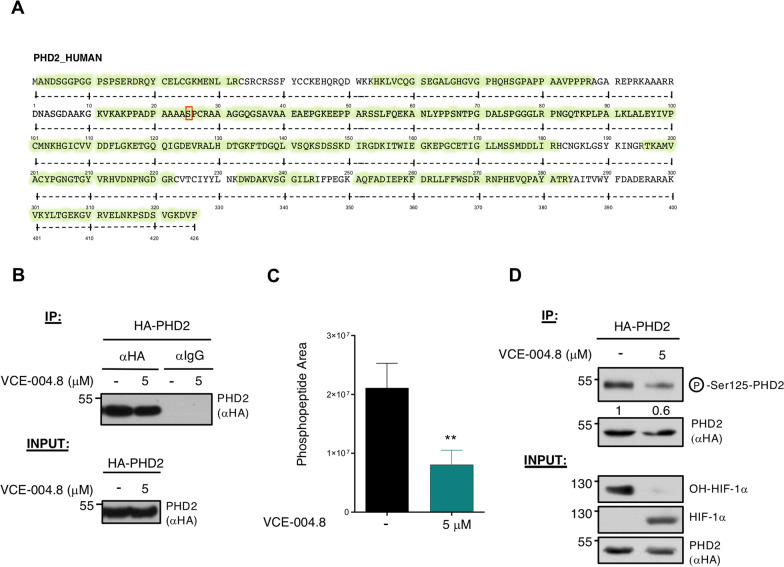
Fig. 1.
VCE-004.8 modulates PHD2 Ser125 phosphorylation. A Schematic representation of mass spectrometry (MS) analysis results. A red box within the PHD2 sequence (101–150) points out phosphorylation found at Ser125 in control experiment, which reduces its signal under VCE-004.8 treatment. B HEK-293T cells were transfected with HA-PHD2 plasmid, treated with 5 μM VCE-004.8 for 24 h and finally lysed. PHD2 protein was immunoprecipitated using either specific antibody (anti-HA) or an unspecific one (rat IgG). Immunoprecipitation representation of the proteomic analyses performed (n = 3). C Immunoprecipitation representation of the proteomic analyses, Ser125 phosphopeptide peak area of the three replicates of the mass spectrometry assay. Data represent the mean ± SD (n = 3) and significance was determined by Unpaired t test. p = 0.0096; **p < 0.01 VCE-004.8 vs Negative control. D HEK-293T cells were transfected with HA-PHD2 plasmid, treated with 5 µM VCE-004.8 for 24 h and lysed. A fraction was subjected to IP using an anti-HA antibody. After elution phosphorylation was revealed with a specific anti-phospho Ser125-PHD2 antibody, while exogenous HA-PHD2 protein levels were visualized with an anti-HA antibody (top panel). The remaining extract fraction was tested to analyze the steady state levels of HIF-1α and its hydroxylated form (lower panel). A representative western blot of three independent analyses is shown