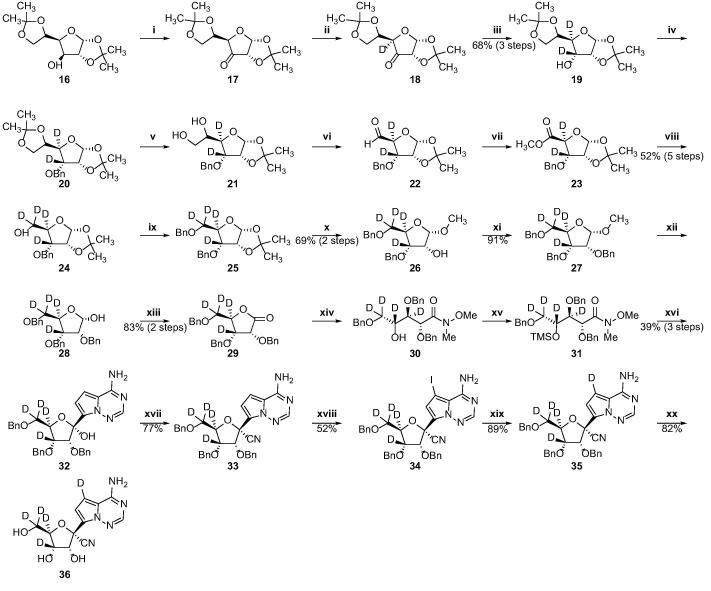
Scheme 4.
Synthesis of the penta-deuterated GS-441524 analog. Reagents and conditions: (i) IBX, acetonitrile, 85 °C, 6.5 h; (ii) D2O, pyridine, 95 °C for 20 min, then 1 day, rt, repeated 3 times (iii) NaBD4, deuterated methanol/anhydrous THF, rt, 2 h; (iv) NaH, BnBr, DMF, rt, 1 h; (v) 80% aqueous acetic acid, 40 °C, 2 h; (vi) NaIO4, ethanol/water, rt, 1 h; (vii) NaHCO3, Br2, methanol/water, rt, 3 h; (viii) NaBD4, anhydrous THF/deuterated methanol, rt, 2 h; (ix) NaH, BnBr, DMF, rt, 2 h; (x) HCl, methanol, 65 °C, 1 h; (xi) NaH, BnBr, DMF, rt, 2 h; (xii) 80% acetic acid aqueous solution, 60 °C, 4 h; (xiii) I2, K2CO3, tert-butanol, 80 °C, 4 h; (xiv) N,O-dimethylhydroxylamine hydrochloride, 2 M i-PrMgCl,anhydrous THF, 0 °C, 1.5 h; (xv) imidazole, TMSCl, DCM, rt, 0.5 h; (xvi) TMSCl, 3 M MeMgBr, 1.3 M i-PrMgCl·LiCl, anhydrous THF, −10 to 0 °C for 1 h, then 0 °C for 1 h; (xvii) TMSOTf, TFMS, TMSCN, DCM, −70 °C, 1 h; (xviii) NIS, TFA, DMF, 50 °C, 1 h; (xix) triethylamine, Pd/C, D2, anhydrous THF, 60 °C, 1 h;(xx) BCl3, DCM, −40 °C, 4 h.